Small-molecule inhibitor of the AP endonuclease 1/REF-1 E3330 inhibits pancreatic cancer cell growth and migration
- PMID: 18645011
- PMCID: PMC3569736
- DOI: 10.1158/1535-7163.MCT-08-0113
Small-molecule inhibitor of the AP endonuclease 1/REF-1 E3330 inhibits pancreatic cancer cell growth and migration
Abstract
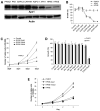
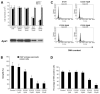
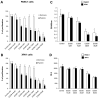
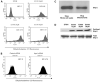
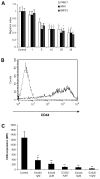
AP endonuclease 1 (APE1; also known as REF-1) contains a DNA repair domain and a redox regulation domain. APE1 is overexpressed in several human cancers, and disruption of APE1 function has detrimental effects on cancer cell viability. However, the selective contribution of the redox and the DNA repair domains to maintenance of cellular homeostasis in cancer has not been elucidated. In the present study, we used E3330, a small-molecule inhibitor of APE1 redox domain function, to interrogate the functional relevance of sustained redox function in pancreatic cancer. We show that E3330 significantly reduces the growth of human pancreatic cancer cells in vitro. This phenomenon was further confirmed by a small interfering RNA experiment to knockdown APE1 expression in pancreatic cancer cells. Further, the growth-inhibitory effects of E3330 are accentuated by hypoxia, and this is accompanied by striking inhibition in the DNA-binding ability of hypoxia-inducible factor-1alpha, a hypoxia-induced transcription factor. E3330 exposure promotes endogenous reactive oxygen species formation in pancreatic cancer cells, and the resulting oxidative stress is associated with higher levels of oxidized, and hence inactive, SHP-2, an essential protein tyrosine phosphatase that promotes cancer cell proliferation in its active state. Finally, E3330 treatment inhibits pancreatic cancer cell migration as assessed by in vitro chemokine assays. E3330 shows anticancer properties at multiple functional levels in pancreatic cancer, such as inhibition of cancer cell growth and migration. Inhibition of the APE1 redox function through pharmacologic means has the potential to become a promising therapeutic strategy in this disease.
Figures
Similar articles
-
Impact of APE1/Ref-1 redox inhibition on pancreatic tumor growth.Mol Cancer Ther. 2011 Sep;10(9):1698-708. doi: 10.1158/1535-7163.MCT-11-0107. Epub 2011 Jun 23. Mol Cancer Ther. 2011. PMID: 21700832 Free PMC article.
-
Functional analysis of novel analogues of E3330 that block the redox signaling activity of the multifunctional AP endonuclease/redox signaling enzyme APE1/Ref-1.Antioxid Redox Signal. 2011 Apr 15;14(8):1387-401. doi: 10.1089/ars.2010.3410. Epub 2011 Jan 4. Antioxid Redox Signal. 2011. PMID: 20874257 Free PMC article.
-
APE1/Ref-1 regulates STAT3 transcriptional activity and APE1/Ref-1-STAT3 dual-_targeting effectively inhibits pancreatic cancer cell survival.PLoS One. 2012;7(10):e47462. doi: 10.1371/journal.pone.0047462. Epub 2012 Oct 19. PLoS One. 2012. PMID: 23094050 Free PMC article.
-
Inhibitors of nuclease and redox activity of apurinic/apyrimidinic endonuclease 1/redox effector factor 1 (APE1/Ref-1).Bioorg Med Chem. 2017 May 1;25(9):2531-2544. doi: 10.1016/j.bmc.2017.01.028. Epub 2017 Jan 21. Bioorg Med Chem. 2017. PMID: 28161249 Review.
-
The DNA base excision repair protein Ape1/Ref-1 as a therapeutic and chemopreventive _target.Mol Aspects Med. 2007 Jun-Aug;28(3-4):375-95. doi: 10.1016/j.mam.2007.04.005. Epub 2007 May 3. Mol Aspects Med. 2007. PMID: 17560642 Review.
Cited by
-
Specific inhibition of the redox activity of ape1/ref-1 by e3330 blocks tnf-α-induced activation of IL-8 production in liver cancer cell lines.PLoS One. 2013 Aug 15;8(8):e70909. doi: 10.1371/journal.pone.0070909. eCollection 2013. PLoS One. 2013. PMID: 23967134 Free PMC article.
-
APE1 Promotes Pancreatic Cancer Proliferation through GFRα1/Src/ERK Axis-Cascade Signaling in Response to GDNF.Int J Mol Sci. 2020 May 19;21(10):3586. doi: 10.3390/ijms21103586. Int J Mol Sci. 2020. PMID: 32438692 Free PMC article.
-
Spiclomazine induces apoptosis associated with the suppression of cell viability, migration and invasion in pancreatic carcinoma cells.PLoS One. 2013 Jun 20;8(6):e66362. doi: 10.1371/journal.pone.0066362. Print 2013. PLoS One. 2013. PMID: 23840452 Free PMC article.
-
Colon cancer progression is driven by APEX1-mediated upregulation of Jagged.J Clin Invest. 2013 Jul 1;123(8):3211-30. doi: 10.1172/JCI65521. Online ahead of print. J Clin Invest. 2013. PMID: 23863623 Free PMC article.
-
The APE1/REF-1 and the hallmarks of cancer.Mol Biol Rep. 2024 Jan 2;51(1):47. doi: 10.1007/s11033-023-08946-9. Mol Biol Rep. 2024. PMID: 38165468 Review.
References
-
- Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat Med. 2004;10:789–99. - PubMed
-
- Harris AL. Hypoxia—a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2:38–47. - PubMed
-
- Fishel ML, Kelley MR. The DNA base excision repair protein Ape1/Ref-1 as a therapeutic and chemopreventive _target. Mol Aspects Med. 2007;28:375–95. - PubMed
-
- Kelley MR, Cheng L, Foster R, et al. Elevated and altered expression of the multifunctional DNA base excision repair and redox enzyme Ape1/ref-1 in prostate cancer. Clin Cancer Res. 2001;7:824–30. - PubMed
-
- Sak SC, Harnden P, Johnston CF, Paul AB, Kiltie AE. APE1 and XRCC1 protein expression levels predict cancer-specific survival following radical radiotherapy in bladder cancer. Clin Cancer Res. 2005;11:6205–11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

