The kinase domain of mitochondrial PINK1 faces the cytoplasm
- PMID: 18687899
- PMCID: PMC2575334
- DOI: 10.1073/pnas.0802814105
The kinase domain of mitochondrial PINK1 faces the cytoplasm
Abstract
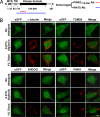
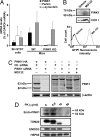
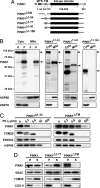
Mutations in PTEN-induced putative kinase 1 (PINK1) are a cause of autosomal recessive familial Parkinson's disease (PD). Efforts in deducing the PINK1 signaling pathway have been hindered by controversy around its subcellular and submitochondrial localization and the authenticity of its reported substrates. We show here that this mitochondrial protein exhibits a topology in which the kinase domain faces the cytoplasm and the N-terminal tail is inside the mitochondria. Although deletion of the transmembrane domain disrupts this topology, common PD-linked PINK1 mutations do not. These results are critical in rectifying the location and orientation of PINK1 in mitochondria, and they should help decipher its normal physiological function and potential pathogenic role in PD.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Differential submitochondrial localization of PINK1 as a molecular switch for mediating distinct mitochondrial signaling pathways.Cell Signal. 2015 Dec;27(12):2543-54. doi: 10.1016/j.cellsig.2015.09.020. Epub 2015 Oct 6. Cell Signal. 2015. PMID: 26436374 Free PMC article.
-
N-degron-mediated degradation and regulation of mitochondrial PINK1 kinase.Curr Genet. 2020 Aug;66(4):693-701. doi: 10.1007/s00294-020-01062-2. Epub 2020 Mar 10. Curr Genet. 2020. PMID: 32157382 Review.
-
Reciprocal Roles of Tom7 and OMA1 during Mitochondrial Import and Activation of PINK1.Mol Cell. 2019 Mar 7;73(5):1028-1043.e5. doi: 10.1016/j.molcel.2019.01.002. Epub 2019 Feb 4. Mol Cell. 2019. PMID: 30733118
-
Cytoplasmic Pink1 activity protects neurons from dopaminergic neurotoxin MPTP.Proc Natl Acad Sci U S A. 2008 Feb 5;105(5):1716-21. doi: 10.1073/pnas.0705363105. Epub 2008 Jan 24. Proc Natl Acad Sci U S A. 2008. PMID: 18218782 Free PMC article.
-
Mitophagy: the latest problem for Parkinson's disease.Trends Mol Med. 2011 Mar;17(3):158-65. doi: 10.1016/j.molmed.2010.11.002. Epub 2010 Dec 9. Trends Mol Med. 2011. PMID: 21146459 Review.
Cited by
-
Parkinson's disease: a complex interplay of mitochondrial DNA alterations and oxidative stress.Int J Mol Sci. 2013 Jan 24;14(2):2388-409. doi: 10.3390/ijms14022388. Int J Mol Sci. 2013. PMID: 23348931 Free PMC article.
-
A Ubl/ubiquitin switch in the activation of Parkin.EMBO J. 2015 Oct 14;34(20):2492-505. doi: 10.15252/embj.201592237. Epub 2015 Aug 7. EMBO J. 2015. PMID: 26254305 Free PMC article.
-
Mitophagy: mechanisms, pathophysiological roles, and analysis.Biol Chem. 2012 Jul;393(7):547-64. doi: 10.1515/hsz-2012-0119. Biol Chem. 2012. PMID: 22944659 Free PMC article. Review.
-
The impact of genetic research on our understanding of Parkinson's disease.Prog Brain Res. 2010;183:21-41. doi: 10.1016/S0079-6123(10)83002-X. Prog Brain Res. 2010. PMID: 20696313 Free PMC article.
-
Parkinson's disease: insights from pathways.Hum Mol Genet. 2010 Apr 15;19(R1):R21-7. doi: 10.1093/hmg/ddq167. Epub 2010 Apr 26. Hum Mol Genet. 2010. PMID: 20421364 Free PMC article. Review.
References
-
- Vila M, Przedborski S. Genetic clues to the pathogenesis of Parkinson's disease. Nat Med. 2004;10(Suppl):S58–S62. - PubMed
-
- Klein C, Lohmann-Hedrich K. Impact of recent genetic findings in Parkinson's disease. Curr Opin Neurol. 2007;20:453–464. - PubMed
-
- Valente EM, et al. Hereditary early-onset Parkinson's disease caused by mutations in PINK1. Science. 2004;304:1158–1160. - PubMed
-
- Deng H, Jankovic J, Guo Y, Xie W, Le W. Small interfering RNA _targeting the PINK1 induces apoptosis in dopaminergic cells SH-SY5Y. Biochem Biophys Res Commun. 2005;337:1133–1138. - PubMed
-
- Petit A, et al. Wild-type PINK1 prevents basal and induced neuronal apoptosis, a protective effect abrogated by Parkinson disease-related mutations. J Biol Chem. 2005;280:34025–34032. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NS11766/NS/NINDS NIH HHS/United States
- R01 AG021617/AG/NIA NIH HHS/United States
- R01 DK058056/DK/NIDDK NIH HHS/United States
- DK58056/DK/NIDDK NIH HHS/United States
- R21 ES013177/ES/NIEHS NIH HHS/United States
- AG21617/AG/NIA NIH HHS/United States
- A608702/PHS HHS/United States
- P01 NS011766/NS/NINDS NIH HHS/United States
- R01 NS042269/NS/NINDS NIH HHS/United States
- NS42269/NS/NINDS NIH HHS/United States
- P50 NS038370/NS/NINDS NIH HHS/United States
- HD83062/HD/NICHD NIH HHS/United States
- NS38370/NS/NINDS NIH HHS/United States
- ES013177/ES/NIEHS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

