Farnesol, a fungal quorum-sensing molecule triggers apoptosis in human oral squamous carcinoma cells
- PMID: 18714396
- PMCID: PMC2517640
- DOI: 10.1593/neo.08444
Farnesol, a fungal quorum-sensing molecule triggers apoptosis in human oral squamous carcinoma cells
Abstract
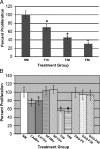
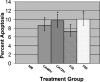
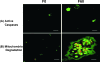
Farnesol is a catabolite within the isoprenoid/cholesterol pathway that has exhibited significant antitumor activity. Farnesol was recently identified as a quorum-sensing molecule produced by the fungal pathogen Candida albicans. In this study, we hypothesize that synthetic and Candida-produced farnesol can induce apoptosis in vitro in oral squamous cell carcinoma (OSCC) lines. Cell proliferation, apoptosis, mitochondrial degradation, and survivin and caspase expressions were examined. In addition, global protein expression profiles were analyzed using proteomic analysis. Results demonstrated significant decrease in proliferation and increase in apoptosis in cells exposed to farnesol and C. albicans culture media. Concurrently, protein expression analysis demonstrated a significant decrease in survivin and an increase in cleaved-caspase expression, whereas fluorescent microscopy revealed the presence of active caspases with mitochondrial degradation in exposed cells. A total of 36 differentially expressed proteins were identified by proteomic analysis. Among the 26 up-regulated proteins were those involved in the inhibition of carcinogenesis, proliferation suppression, and aging. Most notable among the 10 down-regulated proteins were those involved in the inhibition of apoptosis and proteins overexpressed in epithelial carcinomas. This study demonstrates that farnesol significantly inhibits the proliferation of OSCCs and promotes apoptosis in vitro through both the intrinsic and extrinsic apoptotic signaling pathways. In addition, we report for the first time the ability of Candida-produced farnesol to induce a similar apoptotic response through the same pathways. The capability of farnesol to trigger apoptosis in cancer cells makes it a potential tool for studying tumor progression and an attractive candidate as a therapeutic agent.
Figures


Similar articles
-
Farnesol-induced apoptosis in Candida albicans.Antimicrob Agents Chemother. 2009 Jun;53(6):2392-401. doi: 10.1128/AAC.01551-08. Epub 2009 Apr 13. Antimicrob Agents Chemother. 2009. PMID: 19364863 Free PMC article.
-
Antisense-mediated downregulation of anti-apoptotic proteins induces apoptosis and sensitizes head and neck squamous cell carcinoma cells to chemotherapy.Cancer Biol Ther. 2005 Jul;4(7):720-7. doi: 10.4161/cbt.4.7.1783. Epub 2005 Jul 2. Cancer Biol Ther. 2005. PMID: 15917659
-
Suppression of survivin expression in glioblastoma cells by the Ras inhibitor farnesylthiosalicylic acid promotes caspase-dependent apoptosis.Mol Cancer Ther. 2006 Sep;5(9):2337-47. doi: 10.1158/1535-7163.MCT-06-0193. Mol Cancer Ther. 2006. PMID: 16985068
-
Properties and role of the quorum sensing molecule farnesol in relation to the yeast Candida albicans.Pharmazie. 2017 Jun 1;72(6):307-312. doi: 10.1691/ph.2017.6174. Pharmazie. 2017. PMID: 29442016 Review.
-
[Farnesol as a quorum-sensing molecule in Candida albicans].Nihon Ishinkin Gakkai Zasshi. 2008;49(4):281-6. doi: 10.3314/jjmm.49.281. Nihon Ishinkin Gakkai Zasshi. 2008. PMID: 19001754 Review. Japanese.
Cited by
-
Synthesis of Zinc Oxide (ZnO)-Titanium Dioxide (TiO2)-Chitosan-Farnesol Nanocomposites and Assessment of Their Anticancer Potential in Human Leukemic MOLT-4 Cell Line.Bioinorg Chem Appl. 2022 Sep 28;2022:5949086. doi: 10.1155/2022/5949086. eCollection 2022. Bioinorg Chem Appl. 2022. Retraction in: Bioinorg Chem Appl. 2024 Jan 24;2024:9821307. doi: 10.1155/2024/9821307 PMID: 36212987 Free PMC article. Retracted.
-
Farnesol-induced apoptosis in Candida albicans is mediated by Cdr1-p extrusion and depletion of intracellular glutathione.PLoS One. 2011;6(12):e28830. doi: 10.1371/journal.pone.0028830. Epub 2011 Dec 19. PLoS One. 2011. PMID: 22205973 Free PMC article.
-
Candida albicans-epithelial interactions and pathogenicity mechanisms: scratching the surface.Virulence. 2015;6(4):338-46. doi: 10.1080/21505594.2015.1012981. Virulence. 2015. PMID: 25714110 Free PMC article. Review.
-
Quorum sensing by farnesol revisited.Curr Genet. 2017 Oct;63(5):791-797. doi: 10.1007/s00294-017-0683-x. Epub 2017 Feb 28. Curr Genet. 2017. PMID: 28247023 Review.
-
Molecular mechanisms involved in farnesol-induced apoptosis.Cancer Lett. 2010 Jan 28;287(2):123-35. doi: 10.1016/j.canlet.2009.05.015. Epub 2009 Jun 10. Cancer Lett. 2010. PMID: 19520495 Free PMC article. Review.
References
-
- Wright MM, McMaster CR. Phospholipid synthesis, diacylglycerol compartmentation, and apoptosis. Biol Res. 2002;35:223–229. - PubMed
-
- Parkin DM, Bray F, Ferlay J, Pisani P. Global Cancer Statistics, 2002. CA Cancer J Clin. 2005;55:74–108. - PubMed
-
- Enari M, Sakahira H, Yokoyama H, Okawa K, Iwamatsu A, Nagata S. A caspase-activated Dnase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature. 1998;391:43–50. - PubMed
-
- Hengartner MO. Apoptosis: DNA destroyers. Nature. 2001;412:2729. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
