E2F1 inhibits c-Myc-driven apoptosis via PIK3CA/Akt/mTOR and COX-2 in a mouse model of human liver cancer
- PMID: 18722373
- PMCID: PMC2614075
- DOI: 10.1053/j.gastro.2008.07.012
E2F1 inhibits c-Myc-driven apoptosis via PIK3CA/Akt/mTOR and COX-2 in a mouse model of human liver cancer
Abstract
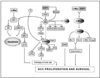
Background & aims: Resistance to apoptosis is essential for cancer growth. We previously reported that hepatic coexpression of c-Myc and E2F1, 2 key regulators of proliferation and apoptosis, enhanced hepatocellular carcinoma (HCC) development in transgenic mice. Here, we investigated the molecular mechanisms underlying oncogenic cooperation between c-Myc and E2F1 in relationship to human liver cancer.
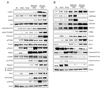
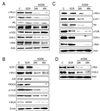
Methods: Activation of pro- and antiapoptotic cascades was assessed by immunoblotting in experimental HCC models and in human HCC. Effect of antisense oligodeoxy nucleotides against c-Myc and E2F1 was studied in human HCC cell lines. Suppression of catalytic subunit p110alpha of phosphatidylinositol 3-kinase (PIK3CA)/Akt, mammalian _target of rapamycin (mTOR), and cyclooxygenase (COX)-2 pathways was achieved by pharmacologic inhibitors and small interfering RNA in human and mouse HCC cell lines.
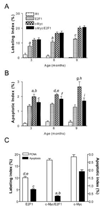
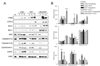
Results: Coexpression with E2F1 did not increase proliferation triggered by c-Myc overexpression but conferred a strong resistance to c-Myc-initiated apoptosis via concomitant induction of PIK3CA/Akt/mTOR and c-Myb/COX-2 survival pathways. COX-2 was not induced in c-Myc and rarely in E2F1 tumors. In human HCC, PIK3CA/Akt/mTOR and c-Myb/COX-2 pathways were similarly activated, with levels of PIK3CA/Akt, mTOR, and c-Myb being inversely associated with patients' survival length. Silencing c-Myc and E2F1 reduced PIK3CA/Akt and mTOR and completely abolished c-Myb and COX-2 expression in human HCC cell lines. Finally, simultaneous inhibition of PIK3CA/Akt/mTOR and COX-2 activity in in vitro models caused massive apoptosis of neoplastic hepatocytes.
Conclusions: E2F1 may function as a critical antiapoptotic factor both in human and in rodent liver cancer through its ability to counteract c-Myc-driven apoptosis via activation of PIK3CA/Akt/mTOR and c-Myb/COX-2 pathways.
Figures


Similar articles
-
AKT (v-akt murine thymoma viral oncogene homolog 1) and N-Ras (neuroblastoma ras viral oncogene homolog) coactivation in the mouse liver promotes rapid carcinogenesis by way of mTOR (mammalian _target of rapamycin complex 1), FOXM1 (forkhead box M1)/SKP2, and c-Myc pathways.Hepatology. 2012 Mar;55(3):833-45. doi: 10.1002/hep.24736. Epub 2011 Dec 19. Hepatology. 2012. PMID: 21993994 Free PMC article.
-
Alpha1-ACT Functions as a Tumour Suppressor in Hepatocellular Carcinoma by Inhibiting the PI3K/AKT/mTOR Signalling Pathway via Activation of PTEN.Cell Physiol Biochem. 2017;41(6):2289-2306. doi: 10.1159/000475648. Epub 2017 Apr 26. Cell Physiol Biochem. 2017. PMID: 28456796
-
4EBP1/eIF4E and p70S6K/RPS6 axes play critical and distinct roles in hepatocarcinogenesis driven by AKT and N-Ras proto-oncogenes in mice.Hepatology. 2015 Jan;61(1):200-13. doi: 10.1002/hep.27396. Epub 2014 Nov 25. Hepatology. 2015. PMID: 25145583 Free PMC article.
-
[EEF1A2 inhibits the p53 function in hepatocellular carcinoma via PI3K/AKT/mTOR-dependent stabilization of MDM4].Pathologe. 2014 Nov;35 Suppl 2:177-84. doi: 10.1007/s00292-014-2007-y. Pathologe. 2014. PMID: 25394965 Review. German.
-
Role of regulatory miRNAs of the PI3K/AKT/mTOR signaling in the pathogenesis of hepatocellular carcinoma.J Cell Physiol. 2020 May;235(5):4146-4152. doi: 10.1002/jcp.29333. Epub 2019 Oct 29. J Cell Physiol. 2020. PMID: 31663122 Review.
Cited by
-
Transcriptome analysis reveals the molecular mechanisms of combined gamma-tocotrienol and hydroxychavicol in preventing the proliferation of 1321N1, SW1783, and LN18 glioma cancer cells.J Physiol Biochem. 2019 Nov;75(4):499-517. doi: 10.1007/s13105-019-00699-z. Epub 2019 Aug 14. J Physiol Biochem. 2019. PMID: 31414341
-
Expression of eukaryotic translation initiation factor 3 subunit B in liver cancer and its prognostic significance.Exp Ther Med. 2020 Jul;20(1):436-446. doi: 10.3892/etm.2020.8726. Epub 2020 May 7. Exp Ther Med. 2020. PMID: 32537008 Free PMC article.
-
Emerging role of MYB transcription factors in cancer drug resistance.Cancer Drug Resist. 2024 Apr 30;7:15. doi: 10.20517/cdr.2023.158. eCollection 2024. Cancer Drug Resist. 2024. PMID: 38835346 Free PMC article. Review.
-
V-ATPase: a master effector of E2F1-mediated lysosomal trafficking, mTORC1 activation and autophagy.Onco_target. 2015 Sep 29;6(29):28057-70. doi: 10.18632/onco_target.4812. Onco_target. 2015. PMID: 26356814 Free PMC article.
-
MiR-15a suppresses hepatocarcinoma cell migration and invasion by directly _targeting cMyb.Am J Transl Res. 2017 Feb 15;9(2):520-532. eCollection 2017. Am J Transl Res. 2017. PMID: 28337280 Free PMC article.
References
-
- Harbour JW, Dean DC. The Rb/E2F pathway: expanding roles and emerging paradigms. Genes Dev. 2000;14:2393–2409. - PubMed
-
- Nevins JR. E2F: a link between the Rb tumor suppressor protein and viral oncoproteins. Science. 1992;258:424–429. - PubMed
-
- Moroy T, Marchio A, Etiemble J, et al. Rearrangement and enhanced expression of c-myc in hepatocellular carcinoma of hepatitis virus infected woodchucks. Nature. 1986;324:276–279. - PubMed
-
- Murakami H, Sanderson ND, Nagy P, et al. Transgenic mouse model for synergistic effects of nuclear oncogenes and growth factors in tumorigenesis: interaction of c-myc and transforming growth factor alpha in hepatic oncogenesis. Cancer Res. 1993;53:1719–1723. - PubMed
-
- Conner EA, Lemmer ER, Omori M, et al. Dual functions of E2F-1 in a transgenic mouse model of liver carcinogenesis. Oncogene. 2000;19:5054–5062. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

