In vivo reconstitution of autophagy in Saccharomyces cerevisiae
- PMID: 18725539
- PMCID: PMC2518709
- DOI: 10.1083/jcb.200801035
In vivo reconstitution of autophagy in Saccharomyces cerevisiae
Abstract
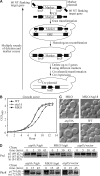
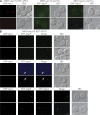
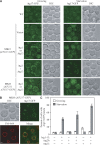
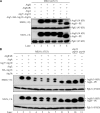
Autophagy is a major intracellular degradative pathway that is involved in various human diseases. The role of autophagy, however, is complex; although the process is generally considered to be cytoprotective, it can also contribute to cellular dysfunction and disease progression. Much progress has been made in our understanding of autophagy, aided in large part by the identification of the autophagy-related (ATG) genes. Nonetheless, our understanding of the molecular mechanism remains limited. In this study, we generated a Saccharomyces cerevisiae multiple-knockout strain with 24 ATG genes deleted, and we used it to carry out an in vivo reconstitution of the autophagy pathway. We determined minimum requirements for different aspects of autophagy and studied the initial protein assembly steps at the phagophore assembly site. In vivo reconstitution enables the study of autophagy within the context of the complex regulatory networks that control this process, an analysis that is not possible with an in vitro system.
Figures

Similar articles
-
New insights into autophagy using a multiple knockout strain.Autophagy. 2008 Nov;4(8):1073-5. doi: 10.4161/auto.6962. Epub 2008 Nov 10. Autophagy. 2008. PMID: 18971623 Free PMC article.
-
Monitoring the Formation of Autophagosomal Precursor Structures in Yeast Saccharomyces cerevisiae.Methods Enzymol. 2017;588:323-365. doi: 10.1016/bs.mie.2016.09.085. Epub 2016 Nov 21. Methods Enzymol. 2017. PMID: 28237109
-
Atg27 is a second transmembrane cycling protein.Autophagy. 2007 May-Jun;3(3):254-6. doi: 10.4161/auto.3823. Epub 2007 May 11. Autophagy. 2007. PMID: 17297289
-
Mechanistic Insights into the Role of Atg11 in Selective Autophagy.J Mol Biol. 2020 Jan 3;432(1):104-122. doi: 10.1016/j.jmb.2019.06.017. Epub 2019 Jun 22. J Mol Biol. 2020. PMID: 31238043 Free PMC article. Review.
-
Atg9 trafficking in the yeast Saccharomyces cerevisiae.Autophagy. 2007 Mar-Apr;3(2):145-8. doi: 10.4161/auto.3608. Epub 2007 Mar 21. Autophagy. 2007. PMID: 17204846 Review.
Cited by
-
Self-interaction is critical for Atg9 transport and function at the phagophore assembly site during autophagy.Mol Biol Cell. 2008 Dec;19(12):5506-16. doi: 10.1091/mbc.e08-05-0544. Epub 2008 Oct 1. Mol Biol Cell. 2008. PMID: 18829864 Free PMC article.
-
A multiple ATG gene knockout strain for yeast two-hybrid analysis.Autophagy. 2009 Jul;5(5):699-705. doi: 10.4161/auto.5.5.8382. Epub 2009 Jul 10. Autophagy. 2009. PMID: 19337029 Free PMC article.
-
The carboxy terminus of yeast Atg13 binds phospholipid membrane via motifs that overlap with the Vac8-interacting domain.Autophagy. 2020 Jun;16(6):1007-1020. doi: 10.1080/15548627.2019.1648117. Epub 2019 Aug 2. Autophagy. 2020. PMID: 31352862 Free PMC article.
-
Autophagy: An Essential Degradation Program for Cellular Homeostasis and Life.Cells. 2018 Dec 19;7(12):278. doi: 10.3390/cells7120278. Cells. 2018. PMID: 30572663 Free PMC article. Review.
-
Peroxisome size provides insights into the function of autophagy-related proteins.Mol Biol Cell. 2009 Sep;20(17):3828-39. doi: 10.1091/mbc.e09-03-0221. Epub 2009 Jul 15. Mol Biol Cell. 2009. PMID: 19605559 Free PMC article.
References
-
- Bowers, K., and T.H. Stevens. 2005. Protein transport from the late Golgi to the vacuole in the yeast Saccharomyces cerevisiae. Biochim. Biophys. Acta. 1744:438–454. - PubMed
-
- Cao, Y., and D.J. Klionsky. 2007. Physiological functions of Atg6/Beclin 1: a unique autophagy-related protein. Cell Res. 17:839–849. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

