Functional expression of IgA receptor FcalphaRI on human platelets
- PMID: 18784345
- PMCID: PMC2614599
- DOI: 10.1189/jlb.0508327
Functional expression of IgA receptor FcalphaRI on human platelets
Abstract
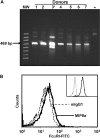
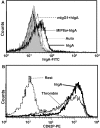
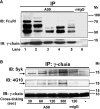
FcalphaRI (CD89) is a human IgA FcR expressed on cells of myeloid lineage such as neutrophils, monocytes, tissue macrophages, eosinophils, and subpopulations of dendritic cells. FcalphaRI mediates cell activation through Src family kinases and downstream tyrosine-based phosphorylation pathways. However, the role of IgA and the expression and role of its cognate receptor FcalphaRI (CD89) in platelet activation are undefined. In the current study, we demonstrate that human platelets express FcalphaRI mRNAs and proteins. Furthermore, we show that the platelet FcalphaRI is associated with the FcR gamma-chain, and cross-linking of FcalphaRI leads to Syk phosphorylation. Clustering of FcalphaRI induces pre-mRNA splicing and protein production of tissue factor and IL-1beta, suggesting novel roles for human platelet FcalphaRI and serum IgA in thrombosis and inflammation.
Figures
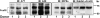

Similar articles
-
IgA Fc receptors.Annu Rev Immunol. 2003;21:177-204. doi: 10.1146/annurev.immunol.21.120601.141011. Epub 2001 Dec 19. Annu Rev Immunol. 2003. PMID: 12524384 Review.
-
Signaling through mutants of the IgA receptor CD89 and consequences for Fc receptor gamma-chain interaction.J Immunol. 2006 Mar 15;176(6):3603-10. doi: 10.4049/jimmunol.176.6.3603. J Immunol. 2006. PMID: 16517729
-
FcalphaRI (CD89) alleles determine the proinflammatory potential of serum IgA.J Immunol. 2007 Mar 15;178(6):3973-82. doi: 10.4049/jimmunol.178.6.3973. J Immunol. 2007. PMID: 17339498 Clinical Trial.
-
Anti-inflammatory role of the IgA Fc receptor (CD89): from autoimmunity to therapeutic perspectives.Autoimmun Rev. 2013 Apr;12(6):666-9. doi: 10.1016/j.autrev.2012.10.011. Epub 2012 Nov 29. Autoimmun Rev. 2013. PMID: 23201915 Review.
-
c-Jun activating binding protein 1 binds to the IgA receptor and modulates protein levels of FcalphaRI and FcRgamma-chain.Eur J Immunol. 2010 Jul;40(7):2035-40. doi: 10.1002/eji.200939985. Eur J Immunol. 2010. PMID: 20411563
Cited by
-
Platelets in Pulmonary Immune Responses and Inflammatory Lung Diseases.Physiol Rev. 2016 Oct;96(4):1211-59. doi: 10.1152/physrev.00038.2015. Epub 2016 Aug 3. Physiol Rev. 2016. PMID: 27489307 Free PMC article. Review.
-
Immunoglobulin A: A next generation of therapeutic antibodies?MAbs. 2011 Jul-Aug;3(4):352-61. doi: 10.4161/mabs.3.4.16092. Epub 2011 Jul 1. MAbs. 2011. PMID: 21691145 Free PMC article. Review.
-
Platelets and Their Interactions with Other Immune Cells.Compr Physiol. 2015 Jul 1;5(3):1265-80. doi: 10.1002/cphy.c140074. Compr Physiol. 2015. PMID: 26140718 Free PMC article. Review.
-
_targeting CD89 on tumor-associated macrophages overcomes resistance to immune checkpoint blockade.J Immunother Cancer. 2022 Dec;10(12):e005447. doi: 10.1136/jitc-2022-005447. J Immunother Cancer. 2022. PMID: 36460336 Free PMC article.
-
The role of platelets in immune-mediated inflammatory diseases.Nat Rev Immunol. 2023 Aug;23(8):495-510. doi: 10.1038/s41577-023-00834-4. Epub 2023 Jan 27. Nat Rev Immunol. 2023. PMID: 36707719 Free PMC article. Review.
References
-
- Monteiro R C, Van De Winkel J G. IgA Fc receptors. Annu Rev Immunol. 2003;21:177–204. - PubMed
-
- Wende H, Colonna M, Ziegler A, Volz A. Organization of the leukocyte receptor cluster (LRC) on human chromosome 19q13.4. Mamm Genome. 1999;10:154–160. - PubMed
-
- Wagtmann N, Rojo S, Eichler E, Mohrenweiser H, Long E O. A new human gene complex encoding the killer cell inhibitory receptors and related monocyte/macrophage receptors. Curr Biol. 1997;7:615–618. - PubMed
-
- Clemetson J M, Polgar J, Magnenat E, Wells T N, Clemetson K J. The platelet collagen receptor glycoprotein VI is a member of the immunoglobulin superfamily closely related to FcαR and the natural killer receptors. J Biol Chem. 1999;274:29019–29024. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- N01AI40068/AI/NIAID NIH HHS/United States
- P30 AR048311/AR/NIAMS NIH HHS/United States
- P30-AR48311/AR/NIAMS NIH HHS/United States
- R01 AR033062/AR/NIAMS NIH HHS/United States
- P01-AR49084/AR/NIAMS NIH HHS/United States
- M01 RR000032/RR/NCRR NIH HHS/United States
- M01-RR00032/RR/NCRR NIH HHS/United States
- R01-AR33062/AR/NIAMS NIH HHS/United States
- R01-AR42476/AR/NIAMS NIH HHS/United States
- R01 AR042476/AR/NIAMS NIH HHS/United States
- P01 AR049084/AR/NIAMS NIH HHS/United States
- C06 RR015490/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

