Vorinostat and sorafenib increase ER stress, autophagy and apoptosis via ceramide-dependent CD95 and PERK activation
- PMID: 18787411
- PMCID: PMC2674577
- DOI: 10.4161/cbt.7.10.6623
Vorinostat and sorafenib increase ER stress, autophagy and apoptosis via ceramide-dependent CD95 and PERK activation
Abstract
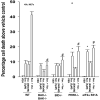
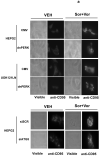
We recently noted that low doses of sorafenib and vorinostat interact in a synergistic fashion to kill carcinoma cells by activating CD95, and this drug combination is entering phase I trials. The present studies mechanistically extended our initial observations. Low doses of sorafenib and vorinostat, but not the individual agents, caused an acidic sphingomyelinase and fumonisin B1-dependent increase in CD95 surface levels and CD95 association with caspase 8. Knock down of CD95 or FADD expression reduced sorafenib/vorinostat lethality. Signaling by CD95 caused PERK activation that was responsible for both promoting caspase 8 association with CD95 and for increased eIF2alpha phosphorylation; suppression of eIF2alpha function abolished drug combination lethality. Cell killing was paralleled by PERK-and eIF2alpha-dependent lowering of c-FLIP-s protein levels and overexpression of c-FLIP-s maintained cell viability. In a CD95-, FADD- and PERK-dependent fashion, sorafenib and vorinostat increased expression of ATG5 that was responsible for enhanced autophagy. Expression of PDGFRbeta and FLT3 were essential for high dose single agent sorafenib treatment to promote autophagy. Suppression of PERK function reduced sorafenib and vorinostat lethality whereas suppression of ATG5 levels elevated sorafenib and vorinostat lethality. Overexpression of c-FLIP-s blocked apoptosis and enhanced drug-induced autophagy. Thus sorafenib and vorinostat promote ceramide-dependent CD95 activation followed by induction of multiple downstream survival regulatory signals: ceramide-CD95-PERK-FADD-pro-caspase 8 (death); ceramide-CD95-PERK-eIF2alpha- downward arrowc-FLIP-s (death); ceramide-CD95-PERK-ATG5-autophagy (survival).
Figures
















Comment in
-
Sorafenib increases endoplasmic reticulum (ER) stress in concert with vorinostat.Cancer Biol Ther. 2011 Dec 15;12(12):1018. doi: 10.4161/cbt.12.12.18135. Epub 2011 Dec 15. Cancer Biol Ther. 2011. PMID: 22095133 No abstract available.
Similar articles
-
Vorinostat and sorafenib synergistically kill tumor cells via FLIP suppression and CD95 activation.Clin Cancer Res. 2008 Sep 1;14(17):5385-99. doi: 10.1158/1078-0432.CCR-08-0469. Clin Cancer Res. 2008. PMID: 18765530 Free PMC article.
-
Vorinostat and sorafenib increase CD95 activation in gastrointestinal tumor cells through a Ca(2+)-de novo ceramide-PP2A-reactive oxygen species-dependent signaling pathway.Cancer Res. 2010 Aug 1;70(15):6313-24. doi: 10.1158/0008-5472.CAN-10-0999. Epub 2010 Jul 14. Cancer Res. 2010. PMID: 20631069 Free PMC article.
-
Sorafenib and vorinostat kill colon cancer cells by CD95-dependent and -independent mechanisms.Mol Pharmacol. 2009 Aug;76(2):342-55. doi: 10.1124/mol.109.056523. Epub 2009 May 29. Mol Pharmacol. 2009. PMID: 19483104 Free PMC article.
-
Sorafenib activates CD95 and promotes autophagy and cell death via Src family kinases in gastrointestinal tumor cells.Mol Cancer Ther. 2010 Aug;9(8):2220-31. doi: 10.1158/1535-7163.MCT-10-0274. Epub 2010 Aug 3. Mol Cancer Ther. 2010. PMID: 20682655 Free PMC article.
-
The role of cell signaling in the crosstalk between autophagy and apoptosis in the regulation of tumor cell survival in response to sorafenib and neratinib.Semin Cancer Biol. 2020 Nov;66:129-139. doi: 10.1016/j.semcancer.2019.10.013. Epub 2019 Oct 20. Semin Cancer Biol. 2020. PMID: 31644944 Free PMC article. Review.
Cited by
-
Inhibition of MCL-1 enhances lapatinib toxicity and overcomes lapatinib resistance via BAK-dependent autophagy.Cancer Biol Ther. 2009 Nov;8(21):2084-96. doi: 10.4161/cbt.8.21.9895. Epub 2009 Nov 21. Cancer Biol Ther. 2009. PMID: 19823038 Free PMC article.
-
PERK-dependent regulation of ceramide synthase 6 and thioredoxin play a key role in mda-7/IL-24-induced killing of primary human glioblastoma multiforme cells.Cancer Res. 2010 Feb 1;70(3):1120-9. doi: 10.1158/0008-5472.CAN-09-4043. Epub 2010 Jan 26. Cancer Res. 2010. PMID: 20103619 Free PMC article.
-
FADD the bad in head and neck cancer.Cancer Biol Ther. 2013 Sep;14(9):780-1. doi: 10.4161/cbt.26151. Epub 2013 Aug 15. Cancer Biol Ther. 2013. PMID: 23974629 Free PMC article.
-
Stress signaling and the shaping of the mammary tissue in development and cancer.Oncogene. 2014 Nov 27;33(48):5483-90. doi: 10.1038/onc.2013.554. Epub 2014 Jan 13. Oncogene. 2014. PMID: 24413078 Free PMC article. Review.
-
Multiple mechanisms mediate resistance to sorafenib in urothelial cancer.Int J Mol Sci. 2014 Nov 7;15(11):20500-17. doi: 10.3390/ijms151120500. Int J Mol Sci. 2014. PMID: 25387078 Free PMC article.
References
-
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. - PubMed
-
- Tsao H. Management of Cutaneous Melanoma. N Engl J Med. 2004;351:998–1012. - PubMed
-
- Dent P. In: MAP kinase pathways in the control of hepatocyte growth, metabolism and survival. Dufour JF, Clavien P-A, editors. Signaling Pathways in Liver Diseases, Springer Press; 2005. pp. 223–238. Chapter 19.
-
- Dent P, Yacoub A, Fisher PB, Hagan MP, Grant S. MAPK pathways in radiation responses. Oncogene. 2003;22:5885–96. - PubMed
-
- Valerie K, Yacoub A, Hagan MP, et al. Radiation-induced cell signaling: inside-out and outside-in. Mol Cancer Ther. 2007;6:789–801. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA108520/CA/NCI NIH HHS/United States
- R01-CA77141/CA/NCI NIH HHS/United States
- R01-CA63753/CA/NCI NIH HHS/United States
- R01 CA063753/CA/NCI NIH HHS/United States
- R01-DK52825/DK/NIDDK NIH HHS/United States
- P01 CA104177/CA/NCI NIH HHS/United States
- R37 GM043880-18/GM/NIGMS NIH HHS/United States
- R01 DK052825/DK/NIDDK NIH HHS/United States
- P01-CA104177/CA/NCI NIH HHS/United States
- R01-CA108520/CA/NCI NIH HHS/United States
- C06 RR018823/RR/NCRR NIH HHS/United States
- C06-RR018823/RR/NCRR NIH HHS/United States
- R01 CA061774/CA/NCI NIH HHS/United States
- R37 GM043880/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
