Hedgehog signaling regulates epithelial-mesenchymal transition during biliary fibrosis in rodents and humans
- PMID: 18802480
- PMCID: PMC2542850
- DOI: 10.1172/JCI35875
Hedgehog signaling regulates epithelial-mesenchymal transition during biliary fibrosis in rodents and humans
Abstract
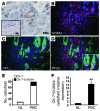
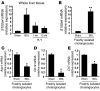
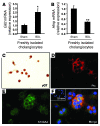
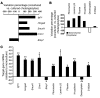
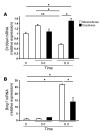
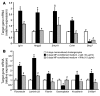
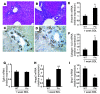
Epithelial-mesenchymal transitions (EMTs) play an important role in tissue construction during embryogenesis, and evidence suggests that this process may also help to remodel some adult tissues after injury. Activation of the hedgehog (Hh) signaling pathway regulates EMT during development. This pathway is also induced by chronic biliary injury, a condition in which EMT has been suggested to have a role. We evaluated the hypothesis that Hh signaling promotes EMT in adult bile ductular cells (cholangiocytes). In liver sections from patients with chronic biliary injury and in primary cholangiocytes isolated from rats that had undergone bile duct ligation (BDL), an experimental model of biliary fibrosis, EMT was localized to cholangiocytes with Hh pathway activity. Relief of ductal obstruction in BDL rats reduced Hh pathway activity, EMT, and biliary fibrosis. In mouse cholangiocytes, coculture with myofibroblastic hepatic stellate cells, a source of soluble Hh ligands, promoted EMT and cell migration. Addition of Hh-neutralizing antibodies to cocultures blocked these effects. Finally, we found that EMT responses to BDL were enhanced in patched-deficient mice, which display excessive activation of the Hh pathway. Together, these data suggest that activation of Hh signaling promotes EMT and contributes to the evolution of biliary fibrosis during chronic cholestasis.
Figures


Comment in
-
Hedgehog signaling in biliary fibrosis.J Clin Invest. 2008 Oct;118(10):3263-5. doi: 10.1172/JCI37189. J Clin Invest. 2008. PMID: 18802487 Free PMC article.
Similar articles
-
Hedgehog signaling in biliary fibrosis.J Clin Invest. 2008 Oct;118(10):3263-5. doi: 10.1172/JCI37189. J Clin Invest. 2008. PMID: 18802487 Free PMC article.
-
The hedgehog pathway regulates remodelling responses to biliary obstruction in rats.Gut. 2008 Sep;57(9):1275-82. doi: 10.1136/gut.2008.148619. Epub 2008 Mar 28. Gut. 2008. PMID: 18375471
-
Hedgehog-mediated mesenchymal-epithelial interactions modulate hepatic response to bile duct ligation.Lab Invest. 2007 May;87(5):499-514. doi: 10.1038/labinvest.3700537. Epub 2007 Mar 5. Lab Invest. 2007. PMID: 17334411
-
Hedgehog signaling in cholangiocytes.Curr Opin Gastroenterol. 2011 May;27(3):268-75. doi: 10.1097/MOG.0b013e32834550b4. Curr Opin Gastroenterol. 2011. PMID: 21423008 Free PMC article. Review.
-
Animal models of biliary tract injury.Curr Opin Gastroenterol. 2012 May;28(3):239-43. doi: 10.1097/MOG.0b013e32835264d9. Curr Opin Gastroenterol. 2012. PMID: 22450892 Review.
Cited by
-
Notch signaling regulates tubular morphogenesis during repair from biliary damage in mice.J Hepatol. 2013 Jul;59(1):124-30. doi: 10.1016/j.jhep.2013.02.025. Epub 2013 Mar 7. J Hepatol. 2013. PMID: 23500150 Free PMC article.
-
Genetic labeling does not detect epithelial-to-mesenchymal transition of cholangiocytes in liver fibrosis in mice.Gastroenterology. 2010 Sep;139(3):987-98. doi: 10.1053/j.gastro.2010.05.005. Epub 2010 Jun 20. Gastroenterology. 2010. PMID: 20546735 Free PMC article.
-
Inhibition of endogenous hedgehog signaling protects against acute liver injury after ischemia reperfusion.Pharm Res. 2010 Nov;27(11):2492-504. doi: 10.1007/s11095-010-0246-z. Epub 2010 Aug 25. Pharm Res. 2010. PMID: 20737284
-
Smad phosphoisoform signals in acute and chronic liver injury: similarities and differences between epithelial and mesenchymal cells.Cell Tissue Res. 2012 Jan;347(1):225-43. doi: 10.1007/s00441-011-1178-6. Epub 2011 May 31. Cell Tissue Res. 2012. PMID: 21626291 Free PMC article. Review.
-
Hedgehog signaling in biliary fibrosis.J Clin Invest. 2008 Oct;118(10):3263-5. doi: 10.1172/JCI37189. J Clin Invest. 2008. PMID: 18802487 Free PMC article.
References
-
- Milani S., et al. Procollagen expression by nonparenchymal rat liver cells in experimental biliary fibrosis. Gastroenterology. 1990;98:175–184. - PubMed
-
- Kinnman N., et al. The myofibroblastic conversion of peribiliary fibrogenic cells distinct from hepatic stellate cells is stimulated by platelet-derived growth factor during liver fibrogenesis. Lab. Invest. 2003;83:163–173. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

