Protein isoaspartate methyltransferase prevents apoptosis induced by oxidative stress in endothelial cells: role of Bcl-Xl deamidation and methylation
- PMID: 18806875
- PMCID: PMC2532751
- DOI: 10.1371/journal.pone.0003258
Protein isoaspartate methyltransferase prevents apoptosis induced by oxidative stress in endothelial cells: role of Bcl-Xl deamidation and methylation
Retraction in
-
Retraction: Protein Isoaspartate Methyltransferase Prevents Apoptosis Induced by Oxidative Stress in Endothelial Cells: Role of Bcl-Xl Deamidation and Methylation.PLoS One. 2018 Nov 8;13(11):e0207530. doi: 10.1371/journal.pone.0207530. eCollection 2018. PLoS One. 2018. PMID: 30408107 Free PMC article. No abstract available.
Abstract
Background: Natural proteins undergo in vivo spontaneous post-biosynthetic deamidation of specific asparagine residues with isoaspartyl formation. Deamidated-isomerized molecules are both structurally and functionally altered. The enzyme isoaspartyl protein carboxyl-O-methyltransferase (PCMT; EC 2.1.1.77) has peculiar substrate specificity towards these deamidated proteins. It catalyzes methyl esterification of the free alpha-carboxyl group at the isoaspartyl site, thus initiating the repair of these abnormal proteins through the conversion of the isopeptide bond into a normal alpha-peptide bond. Deamidation occurs slowly during cellular and molecular aging, being accelerated by physical-chemical stresses brought to the living cells. Previous evidence supports a role of protein deamidation in the acquisition of susceptibility to apoptosis. Aim of this work was to shed a light on the role of PCMT in apoptosis clarifying the relevant mechanism(s).
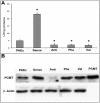
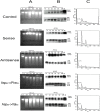
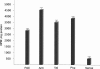
Methodology/principal findings: Endothelial cells transiently transfected with various constructs of PCMT, i.e. overexpressing wild type PCMT or negative dominants, were used to investigate the role of protein methylation during apoptosis induced by oxidative stress (H(2)O(2); 0.1-0.5 mM range). Results show that A) Cells overexpressing "wild type" human PCMT were resistant to apoptosis, whereas overexpression of antisense PCMT induces high sensitivity to apoptosis even at low H(2)O(2) concentrations. B) PCMT protective effect is specifically due to its methyltransferase activity rather than to any other non-enzymatic interactions. In fact negative dominants, overexpressing PCMT mutants devoid of catalytic activity do not prevent apoptosis. C) Cells transfected with antisense PCMT, or overexpressing a PCMT mutant, accumulate isoaspartyl-containing damaged proteins upon H(2)O(2) treatment. Proteomics allowed the identification of proteins, which are both PCMT substrates and apoptosis effectors, whose deamidation occurs under oxidative stress conditions leading to programmed cell death. These proteins, including Hsp70, Hsp90, actin, and Bcl-xL, are recognized and methylated by PCMT, according to the general repair mechanism of this methyltransferase.
Conclusion/significance: Apoptosis can be modulated by "on/off" switch partitioning the amount of specific protein effectors, which are either in their active (native) or inactive (deamidated) molecular forms. Deamidated proteins can also be functionally restored through methylation. Bcl-xL provides a case for the role of PCMT in the maintenance of functional stability of this antiapoptotic protein.
Conflict of interest statement
Figures

Similar articles
-
Increased methyl esterification of altered aspartyl residues in erythrocyte membrane proteins in response to oxidative stress.Eur J Biochem. 2000 Jul;267(14):4397-405. doi: 10.1046/j.1432-1327.2000.01485.x. Eur J Biochem. 2000. PMID: 10880963
-
Stoichiometric methylation of porcine adrenocorticotropin by protein carboxyl methyltransferase requires deamidation of asparagine 25. Evidence for methylation at the alpha-carboxyl group of atypical L-isoaspartyl residues.J Biol Chem. 1984 Sep 10;259(17):10714-21. J Biol Chem. 1984. PMID: 6088513
-
Methylation of atypical protein aspartyl residues during the stress response of HeLa cells.J Cell Physiol. 1992 Nov;153(2):297-304. doi: 10.1002/jcp.1041530209. J Cell Physiol. 1992. PMID: 1429850
-
Damaged proteins bearing L-isoaspartyl residues and aging: a dynamic equilibrium between generation of isomerized forms and repair by PIMT.Curr Aging Sci. 2011 Feb;4(1):8-18. Curr Aging Sci. 2011. PMID: 21204776 Review.
-
PIMT-Mediated Protein Repair: Mechanism and Implications.Biochemistry (Mosc). 2019 May;84(5):453-463. doi: 10.1134/S0006297919050018. Biochemistry (Mosc). 2019. PMID: 31234761 Review.
Cited by
-
Homocysteinylated albumin promotes increased monocyte-endothelial cell adhesion and up-regulation of MCP1, Hsp60 and ADAM17.PLoS One. 2012;7(2):e31388. doi: 10.1371/journal.pone.0031388. Epub 2012 Feb 3. PLoS One. 2012. Retraction in: PLoS One. 2019 Apr 12;14(4):e0215587. doi: 10.1371/journal.pone.0215587 PMID: 22319627 Free PMC article. Retracted.
-
The microRNA 15a/16-1 cluster down-regulates protein repair isoaspartyl methyltransferase in hepatoma cells: implications for apoptosis regulation.J Biol Chem. 2011 Dec 23;286(51):43690-43700. doi: 10.1074/jbc.M111.290437. Epub 2011 Oct 27. J Biol Chem. 2011. Retraction in: J Biol Chem. 2019 May 24;294(21):8350. doi: 10.1074/jbc.RX119.009146 PMID: 22033921 Free PMC article. Retracted.
-
A comprehensive view of the epigenetic landscape. Part II: Histone post-translational modification, nucleosome level, and chromatin regulation by ncRNAs.Neurotox Res. 2015 Feb;27(2):172-97. doi: 10.1007/s12640-014-9508-6. Epub 2014 Dec 17. Neurotox Res. 2015. PMID: 25516120 Free PMC article. Review.
-
Protein L-isoaspartyl methyltransferase regulates p53 activity.Nat Commun. 2012 Jun 26;3:927. doi: 10.1038/ncomms1933. Nat Commun. 2012. PMID: 22735455 Free PMC article.
-
Defective responses to oxidative stress in protein l-isoaspartyl repair-deficient Caenorhabditis elegans.Mech Ageing Dev. 2009 Oct;130(10):670-80. doi: 10.1016/j.mad.2009.08.002. Epub 2009 Aug 12. Mech Ageing Dev. 2009. PMID: 19682488 Free PMC article.
References
-
- Clarke S. Aging as war between chemical and biochemical processes: protein methylation and the recognition of age-damaged proteins for repair. Ageing Res Rev. 2003;2:263–285. - PubMed
-
- Aswad DW, Paranadi MV, Schurter BT. Isoaspartate in peptides and proteins: formation, significance, and analysis. J Pharm Biomed Anal. 2000;21:1129–1136. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

