Protein isoaspartate methyltransferase prevents apoptosis induced by oxidative stress in endothelial cells: role of Bcl-Xl deamidation and methylation
- PMID: 18806875
- PMCID: PMC2532751
- DOI: 10.1371/journal.pone.0003258
Protein isoaspartate methyltransferase prevents apoptosis induced by oxidative stress in endothelial cells: role of Bcl-Xl deamidation and methylation
Retraction in
-
Retraction: Protein Isoaspartate Methyltransferase Prevents Apoptosis Induced by Oxidative Stress in Endothelial Cells: Role of Bcl-Xl Deamidation and Methylation.PLoS One. 2018 Nov 8;13(11):e0207530. doi: 10.1371/journal.pone.0207530. eCollection 2018. PLoS One. 2018. PMID: 30408107 Free PMC article. No abstract available.
Abstract
Background: Natural proteins undergo in vivo spontaneous post-biosynthetic deamidation of specific asparagine residues with isoaspartyl formation. Deamidated-isomerized molecules are both structurally and functionally altered. The enzyme isoaspartyl protein carboxyl-O-methyltransferase (PCMT; EC 2.1.1.77) has peculiar substrate specificity towards these deamidated proteins. It catalyzes methyl esterification of the free alpha-carboxyl group at the isoaspartyl site, thus initiating the repair of these abnormal proteins through the conversion of the isopeptide bond into a normal alpha-peptide bond. Deamidation occurs slowly during cellular and molecular aging, being accelerated by physical-chemical stresses brought to the living cells. Previous evidence supports a role of protein deamidation in the acquisition of susceptibility to apoptosis. Aim of this work was to shed a light on the role of PCMT in apoptosis clarifying the relevant mechanism(s).
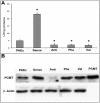
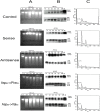
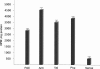
Methodology/principal findings: Endothelial cells transiently transfected with various constructs of PCMT, i.e. overexpressing wild type PCMT or negative dominants, were used to investigate the role of protein methylation during apoptosis induced by oxidative stress (H(2)O(2); 0.1-0.5 mM range). Results show that A) Cells overexpressing "wild type" human PCMT were resistant to apoptosis, whereas overexpression of antisense PCMT induces high sensitivity to apoptosis even at low H(2)O(2) concentrations. B) PCMT protective effect is specifically due to its methyltransferase activity rather than to any other non-enzymatic interactions. In fact negative dominants, overexpressing PCMT mutants devoid of catalytic activity do not prevent apoptosis. C) Cells transfected with antisense PCMT, or overexpressing a PCMT mutant, accumulate isoaspartyl-containing damaged proteins upon H(2)O(2) treatment. Proteomics allowed the identification of proteins, which are both PCMT substrates and apoptosis effectors, whose deamidation occurs under oxidative stress conditions leading to programmed cell death. These proteins, including Hsp70, Hsp90, actin, and Bcl-xL, are recognized and methylated by PCMT, according to the general repair mechanism of this methyltransferase.
Conclusion/significance: Apoptosis can be modulated by "on/off" switch partitioning the amount of specific protein effectors, which are either in their active (native) or inactive (deamidated) molecular forms. Deamidated proteins can also be functionally restored through methylation. Bcl-xL provides a case for the role of PCMT in the maintenance of functional stability of this antiapoptotic protein.
Conflict of interest statement
Figures

Similar articles
-
Increased methyl esterification of altered aspartyl residues in erythrocyte membrane proteins in response to oxidative stress.Eur J Biochem. 2000 Jul;267(14):4397-405. doi: 10.1046/j.1432-1327.2000.01485.x. Eur J Biochem. 2000. PMID: 10880963
-
Stoichiometric methylation of porcine adrenocorticotropin by protein carboxyl methyltransferase requires deamidation of asparagine 25. Evidence for methylation at the alpha-carboxyl group of atypical L-isoaspartyl residues.J Biol Chem. 1984 Sep 10;259(17):10714-21. J Biol Chem. 1984. PMID: 6088513
-
Methylation of atypical protein aspartyl residues during the stress response of HeLa cells.J Cell Physiol. 1992 Nov;153(2):297-304. doi: 10.1002/jcp.1041530209. J Cell Physiol. 1992. PMID: 1429850
-
Damaged proteins bearing L-isoaspartyl residues and aging: a dynamic equilibrium between generation of isomerized forms and repair by PIMT.Curr Aging Sci. 2011 Feb;4(1):8-18. Curr Aging Sci. 2011. PMID: 21204776 Review.
-
PIMT-Mediated Protein Repair: Mechanism and Implications.Biochemistry (Mosc). 2019 May;84(5):453-463. doi: 10.1134/S0006297919050018. Biochemistry (Mosc). 2019. PMID: 31234761 Review.
Cited by
-
Control of cellular Bcl-xL levels by deamidation-regulated degradation.PLoS Biol. 2013;11(6):e1001588. doi: 10.1371/journal.pbio.1001588. Epub 2013 Jun 25. PLoS Biol. 2013. PMID: 23823868 Free PMC article.
-
Assessing analytical methods to monitor isoAsp formation in monoclonal antibodies.Front Pharmacol. 2014 Apr 29;5:87. doi: 10.3389/fphar.2014.00087. eCollection 2014. Front Pharmacol. 2014. PMID: 24808864 Free PMC article.
-
N52 monodeamidated Bcl‑xL shows impaired oncogenic properties in vivo and in vitro.Onco_target. 2016 Mar 29;7(13):17129-43. doi: 10.18632/onco_target.7938. Onco_target. 2016. PMID: 26958941 Free PMC article.
-
The enzyme L-isoaspartyl (D-aspartyl) methyltransferase is required for VEGF-dependent endothelial cell migration and tubulogenesis.Mol Cell Biochem. 2016 Feb;413(1-2):37-46. doi: 10.1007/s11010-015-2637-2. Epub 2016 Jan 6. Mol Cell Biochem. 2016. PMID: 26738492
-
Protein-L-isoaspartate (D-aspartate) O-methyltransferase protects cardiomyocytes against hypoxia induced apoptosis through inhibiting proapoptotic kinase Mst1.Int J Cardiol. 2013 Oct 9;168(4):3291-9. doi: 10.1016/j.ijcard.2013.04.045. Epub 2013 May 3. Int J Cardiol. 2013. PMID: 23647599 Free PMC article.
References
-
- Clarke S. Aging as war between chemical and biochemical processes: protein methylation and the recognition of age-damaged proteins for repair. Ageing Res Rev. 2003;2:263–285. - PubMed
-
- Aswad DW, Paranadi MV, Schurter BT. Isoaspartate in peptides and proteins: formation, significance, and analysis. J Pharm Biomed Anal. 2000;21:1129–1136. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

