Self-interaction is critical for Atg9 transport and function at the phagophore assembly site during autophagy
- PMID: 18829864
- PMCID: PMC2592676
- DOI: 10.1091/mbc.e08-05-0544
Self-interaction is critical for Atg9 transport and function at the phagophore assembly site during autophagy
Abstract
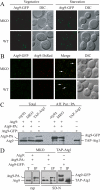
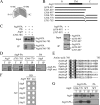
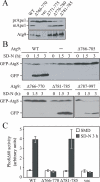
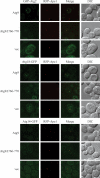
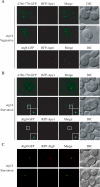
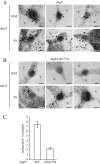
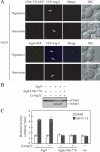
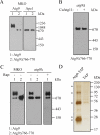
Autophagy is the degradation of a cell's own components within lysosomes (or the analogous yeast vacuole), and its malfunction contributes to a variety of human diseases. Atg9 is the sole integral membrane protein required in formation of the initial sequestering compartment, the phagophore, and is proposed to play a key role in membrane transport; the phagophore presumably expands by vesicular addition to form a complete autophagosome. It is not clear through what mechanism Atg9 functions at the phagophore assembly site (PAS). Here we report that Atg9 molecules self-associate independently of other known autophagy proteins in both nutrient-rich and starvation conditions. Mutational analyses reveal that self-interaction is critical for anterograde transport of Atg9 to the PAS. The ability of Atg9 to self-interact is required for both selective and nonselective autophagy at the step of phagophore expansion at the PAS. Our results support a model in which Atg9 multimerization facilitates membrane flow to the PAS for phagophore formation.
Figures
Similar articles
-
Atg9 trafficking in autophagy-related pathways.Autophagy. 2007 May-Jun;3(3):271-4. doi: 10.4161/auto.3912. Epub 2007 May 29. Autophagy. 2007. PMID: 17329962
-
Double duty of Atg9 self-association in autophagosome biogenesis.Autophagy. 2009 Apr;5(3):385-7. doi: 10.4161/auto.5.3.7699. Epub 2009 Apr 23. Autophagy. 2009. PMID: 19182520 Free PMC article.
-
Phosphorylation of Atg9 regulates movement to the phagophore assembly site and the rate of autophagosome formation.Autophagy. 2016;12(4):648-58. doi: 10.1080/15548627.2016.1157237. Autophagy. 2016. PMID: 27050455 Free PMC article.
-
Transcriptional regulation of ATG9 by the Pho23-Rpd3 complex modulates the frequency of autophagosome formation.Autophagy. 2014 Sep;10(9):1681-2. doi: 10.4161/auto.29641. Epub 2014 Jul 7. Autophagy. 2014. PMID: 25046109 Free PMC article. Review.
-
Atg9 trafficking in the yeast Saccharomyces cerevisiae.Autophagy. 2007 Mar-Apr;3(2):145-8. doi: 10.4161/auto.3608. Epub 2007 Mar 21. Autophagy. 2007. PMID: 17204846 Review.
Cited by
-
Computational model for autophagic vesicle dynamics in single cells.Autophagy. 2013 Jan;9(1):74-92. doi: 10.4161/auto.22532. Epub 2012 Nov 29. Autophagy. 2013. PMID: 23196898 Free PMC article.
-
ATG16L1 autophagy pathway regulates BAX protein levels and programmed cell death.J Biol Chem. 2020 Oct 30;295(44):15045-15053. doi: 10.1074/jbc.RA120.013999. Epub 2020 Aug 26. J Biol Chem. 2020. PMID: 32848017 Free PMC article.
-
Survival by self-destruction: a role for autophagy in the placenta?Placenta. 2012 Aug;33(8):591-8. doi: 10.1016/j.placenta.2012.04.011. Epub 2012 May 30. Placenta. 2012. PMID: 22652048 Free PMC article. Review.
-
ATG9 vesicles comprise the seed membrane of mammalian autophagosomes.J Cell Biol. 2023 Jul 3;222(7):e202208088. doi: 10.1083/jcb.202208088. Epub 2023 Apr 28. J Cell Biol. 2023. PMID: 37115958 Free PMC article.
-
The Atg1-kinase complex tethers Atg9-vesicles to initiate autophagy.Nat Commun. 2016 Jan 12;7:10338. doi: 10.1038/ncomms10338. Nat Commun. 2016. PMID: 26753620 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

