Preferential cytotoxicity of bortezomib toward hypoxic tumor cells via overactivation of endoplasmic reticulum stress pathways
- PMID: 19010906
- PMCID: PMC3617567
- DOI: 10.1158/0008-5472.CAN-08-2873
Preferential cytotoxicity of bortezomib toward hypoxic tumor cells via overactivation of endoplasmic reticulum stress pathways
Abstract
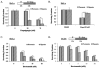
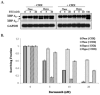
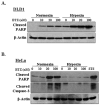
Hypoxia is a dynamic feature of the tumor microenvironment that contributes to drug resistance and cancer progression. We previously showed that components of the unfolded protein response (UPR), elicited by endoplasmic reticulum (ER) stress, are also activated by hypoxia in vitro and in vivo animal and human patient tumors. Here, we report that ER stressors, such as thapsigargin or the clinically used proteasome inhibitor bortezomib, exhibit significantly higher cytotoxicity toward hypoxic compared with normoxic tumor cells, which is accompanied by enhanced activation of UPR effectors in vitro and UPR reporter activity in vivo. Treatment of cells with the translation inhibitor cycloheximide, which relieves ER load, ameliorated this enhanced cytotoxicity, indicating that the increased cytotoxicity is ER stress-dependent. The mode of cell death was cell type-dependent, because DLD1 colorectal carcinoma cells exhibited enhanced apoptosis, whereas HeLa cervical carcinoma cells activated autophagy, blocked apoptosis, and eventually led to necrosis. Pharmacologic or genetic ablation of autophagy increased the levels of apoptosis. These results show that hypoxic tumor cells, which are generally more resistant to genotoxic agents, are hypersensitive to proteasome inhibitors and suggest that combining bortezomib with therapies that _target the normoxic fraction of human tumors can lead to more effective tumor control.
Figures



Similar articles
-
Bortezomib blocks the catabolic process of autophagy via a cathepsin-dependent mechanism, affects endoplasmic reticulum stress and induces caspase-dependent cell death in antiestrogen-sensitive and resistant ER+ breast cancer cells.Autophagy. 2010 Jan;6(1):19-35. doi: 10.4161/auto.6.1.10323. Autophagy. 2010. PMID: 20110775
-
Ritonavir induces endoplasmic reticulum stress and sensitizes sarcoma cells toward bortezomib-induced apoptosis.Mol Cancer Ther. 2008 Jul;7(7):1940-8. doi: 10.1158/1535-7163.MCT-07-2375. Mol Cancer Ther. 2008. PMID: 18645004
-
Bortezomib inhibits PKR-like endoplasmic reticulum (ER) kinase and induces apoptosis via ER stress in human pancreatic cancer cells.Cancer Res. 2005 Dec 15;65(24):11510-9. doi: 10.1158/0008-5472.CAN-05-2394. Cancer Res. 2005. PMID: 16357160
-
_targeting endoplasmic reticulum signaling pathways in cancer.Acta Oncol. 2012 Sep;51(7):822-30. doi: 10.3109/0284186X.2012.689113. Epub 2012 Jun 11. Acta Oncol. 2012. PMID: 22686473 Review.
-
Role of ATF4 in regulation of autophagy and resistance to drugs and hypoxia.Cell Cycle. 2009 Dec;8(23):3838-47. doi: 10.4161/cc.8.23.10086. Epub 2009 Dec 15. Cell Cycle. 2009. PMID: 19887912 Review.
Cited by
-
Celecoxib enhances radiosensitivity of hypoxic glioblastoma cells through endoplasmic reticulum stress.Neuro Oncol. 2013 Sep;15(9):1186-99. doi: 10.1093/neuonc/not062. Epub 2013 May 7. Neuro Oncol. 2013. PMID: 23658321 Free PMC article.
-
Autophagy modulation as a _target for anticancer drug discovery.Acta Pharmacol Sin. 2013 May;34(5):612-24. doi: 10.1038/aps.2013.23. Epub 2013 Apr 8. Acta Pharmacol Sin. 2013. PMID: 23564085 Free PMC article. Review.
-
[Bortezomib and obatoclax for dual blockade of protein degradation pathways show synergistic anti-tumor effect in human acute T lymphoblastic leukemia cells].Nan Fang Yi Ke Da Xue Xue Bao. 2019 Apr 30;39(4):401-408. doi: 10.12122/j.issn.1673-4254.2019.04.04. Nan Fang Yi Ke Da Xue Xue Bao. 2019. PMID: 31068282 Free PMC article. Chinese.
-
Proteasome inhibitor-induced autophagy in PC12 cells overexpressing A53T mutant α-synuclein.Mol Med Rep. 2015 Mar;11(3):1655-60. doi: 10.3892/mmr.2014.3011. Epub 2014 Nov 27. Mol Med Rep. 2015. PMID: 25434876 Free PMC article.
-
A Systematic Review of miR-29 in Cancer.Mol Ther Oncolytics. 2018 Dec 31;12:173-194. doi: 10.1016/j.omto.2018.12.011. eCollection 2019 Mar 29. Mol Ther Oncolytics. 2018. PMID: 30788428 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

