Anandamide-induced behavioral disruption through a vanilloid-dependent mechanism in rats
- PMID: 19015836
- PMCID: PMC2695254
- DOI: 10.1007/s00213-008-1399-x
Anandamide-induced behavioral disruption through a vanilloid-dependent mechanism in rats
Abstract
Rationale: Endocannabinoids are involved in a variety of behavioral and physiological processes that are just beginning to be understood. In the five-choice serial reaction-time task, exogenous cannabinoids have been found to alter attention, but endocannabinoids such as anandamide have not been studied.
Objectives: We used this task to evaluate the effects of anandamide in rats. Since anandamide is a ligand for not only cannabinoid receptors but also transient receptor potential vanilloid 1 (TRPV1) receptors, and as recently suggested, peroxisome proliferator-activated nuclear receptor-alpha (PPARalpha), we also determined whether anandamide's effects in this task were mediated by each of these receptors.
Materials and methods: Whenever one of five holes was illuminated for 2 s, a food pellet was delivered if a response occurred in that hole during the light or within 2 s after the light.
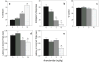
Results: Anandamide increased omission errors and decreased responding during inter-trial intervals. These effects were blocked by the TRPV1 antagonist capsazepine, but not by the cannabinoid-receptor antagonist rimonabant or the PPARalpha antagonist MK886. Testing with open-field activity and food-consumption procedures in the same rats suggested that the disruption of operant responding observed in the attention task was not due to motor depression, anxiety, decreased appetite, or an inability to find and consume food pellets.
Conclusions: The vanilloid-dependent behavioral disruption induced by anandamide was specific to the operant attention task. These effects of anandamide resemble effects of systemically administered dopamine antagonists and might reflect changes in vanilloid-mediated dopamine transmission.
Figures
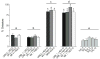

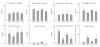
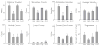
Similar articles
-
Involvement of vanilloid-like receptors in the effects of anandamide on motor behavior and nigrostriatal dopaminergic activity: in vivo and in vitro evidence.Brain Res. 2004 May 8;1007(1-2):152-9. doi: 10.1016/j.brainres.2004.02.016. Brain Res. 2004. PMID: 15064146
-
Anandamide improves the impaired nitric oxide-mediated neurogenic relaxation of the corpus cavernosum in diabetic rats: involvement of cannabinoid CB1 and vanilloid VR1 receptors.BJU Int. 2007 Dec;100(6):1385-90. doi: 10.1111/j.1464-410X.2007.07180.x. Epub 2007 Sep 10. BJU Int. 2007. PMID: 17850365
-
Anandamide capacitates bull spermatozoa through CB1 and TRPV1 activation.PLoS One. 2011 Feb 11;6(2):e16993. doi: 10.1371/journal.pone.0016993. PLoS One. 2011. PMID: 21347292 Free PMC article.
-
Anandamide and the vanilloid receptor (TRPV1).Vitam Horm. 2009;81:389-419. doi: 10.1016/S0083-6729(09)81015-7. Vitam Horm. 2009. PMID: 19647120 Review.
-
Biochemistry and pharmacology of endovanilloids.Pharmacol Ther. 2007 Apr;114(1):13-33. doi: 10.1016/j.pharmthera.2007.01.005. Epub 2007 Feb 2. Pharmacol Ther. 2007. PMID: 17349697 Review.
Cited by
-
Thermoregulatory phenotype of the Trpv1 knockout mouse: thermoeffector dysbalance with hyperkinesis.J Neurosci. 2011 Feb 2;31(5):1721-33. doi: 10.1523/JNEUROSCI.4671-10.2011. J Neurosci. 2011. PMID: 21289181 Free PMC article.
-
Pharmacological Blockade of PPARα Exacerbates Inflammatory Pain-Related Impairment of Spatial Memory in Rats.Biomedicines. 2021 May 27;9(6):610. doi: 10.3390/biomedicines9060610. Biomedicines. 2021. PMID: 34072060 Free PMC article.
-
Dissimilar cannabinoid substitution patterns in mice trained to discriminate Δ(9)-tetrahydrocannabinol or methanandamide from vehicle.Behav Pharmacol. 2011 Sep;22(5-6):480-8. doi: 10.1097/FBP.0b013e328348eced. Behav Pharmacol. 2011. PMID: 21712709 Free PMC article.
-
In vivo pharmacology of endocannabinoids and their metabolic inhibitors: therapeutic implications in Parkinson's disease and abuse liability.Prostaglandins Other Lipid Mediat. 2010 Apr;91(3-4):90-103. doi: 10.1016/j.prostaglandins.2009.05.004. Epub 2009 Jun 10. Prostaglandins Other Lipid Mediat. 2010. PMID: 19523530 Free PMC article. Review.
-
Effects of fatty acid amide hydrolase (FAAH) inhibitors on working memory in rats.Psychopharmacology (Berl). 2016 May;233(10):1879-88. doi: 10.1007/s00213-015-4140-6. Epub 2015 Nov 12. Psychopharmacology (Berl). 2016. PMID: 26558620 Free PMC article.
References
-
- Arguello PA, Jentsch JD. Cannabinoid CB1 receptor-mediated impairment of visuospatial attention in the rat. Psychopharmacology. 2004;177:141–50. - PubMed
-
- Arizzi MN, Cervone KM, Aberman JE, Betz A, Liu Q, Lin S, Makriyannis A, Salamone JD. Behavioral effects of inhibition of cannabinoid metabolism: The amidase inhibitor AM374 enhances the suppression of lever pressing produced by exogenously administered anandamide. Life Sci. 2004;74:1001–11. - PubMed
-
- Berridge KC. Food reward: brain substrates of wanting and liking. Neurosci Biobehav Rev. 1996;20:1–25. - PubMed
-
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–9. - PubMed
-
- Di Marzo V, Lastres-Becker I, Bisogno T, De Petrocellis L, Milone A, Davis JB, Fernandez-Ruiz JJ. Hypolocomotor effects in rats of capsaicin and two long chain capsaicin homologues. Eur J Pharmacol. 2001;420:123–31. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

