Undue reliance on I(2) in assessing heterogeneity may mislead
- PMID: 19036172
- PMCID: PMC2648991
- DOI: 10.1186/1471-2288-8-79
Undue reliance on I(2) in assessing heterogeneity may mislead
Abstract
Background: The heterogeneity statistic I(2), interpreted as the percentage of variability due to heterogeneity between studies rather than sampling error, depends on precision, that is, the size of the studies included.
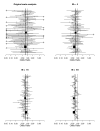
Methods: Based on a real meta-analysis, we simulate artificially 'inflating' the sample size under the random effects model. For a given inflation factor M = 1, 2, 3,... and for each trial i, we create a M-inflated trial by drawing a treatment effect estimate from the random effects model, using s(i)(2)/M as within-trial sampling variance.
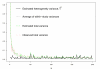
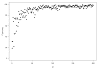
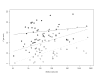
Results: As precision increases, while estimates of the heterogeneity variance tau(2) remain unchanged on average, estimates of I(2) increase rapidly to nearly 100%. A similar phenomenon is apparent in a sample of 157 meta-analyses.
Conclusion: When deciding whether or not to pool treatment estimates in a meta-analysis, the yard-stick should be the clinical relevance of any heterogeneity present. tau(2), rather than I(2), is the appropriate measure for this purpose.
Figures
Similar articles
-
Subgroup analyses in randomised controlled trials: quantifying the risks of false-positives and false-negatives.Health Technol Assess. 2001;5(33):1-56. doi: 10.3310/hta5330. Health Technol Assess. 2001. PMID: 11701102 Review.
-
Controlling type 1 error rate for sequential, bioequivalence studies with crossover designs.Pharm Stat. 2019 Jan;18(1):96-105. doi: 10.1002/pst.1911. Epub 2018 Oct 29. Pharm Stat. 2019. PMID: 30370634
-
On adaptive error spending approach for group sequential trials with random information levels.J Biopharm Stat. 2012;22(4):687-99. doi: 10.1080/10543406.2012.678232. J Biopharm Stat. 2012. PMID: 22651109
-
Comparison of Time-to-First Event and Recurrent-Event Methods in Randomized Clinical Trials.Circulation. 2018 Aug 7;138(6):570-577. doi: 10.1161/CIRCULATIONAHA.117.033065. Circulation. 2018. PMID: 29588314
-
Sample size calculations for randomized controlled trials.Epidemiol Rev. 2002;24(1):39-53. doi: 10.1093/epirev/24.1.39. Epidemiol Rev. 2002. PMID: 12119854 Review. No abstract available.
Cited by
-
Effect of Laryngeal Mask Airway Insertion on Intraocular Pressure Response: Systematic Review and Meta-Analysis.Anesthesiol Res Pract. 2020 Jul 9;2020:7858434. doi: 10.1155/2020/7858434. eCollection 2020. Anesthesiol Res Pract. 2020. PMID: 32695160 Free PMC article. Review.
-
Attitude towards assisted reproductive technology: acceptance of donors eggs, sperms, and embryos as treatment of human infertility: a systematic review and meta-analysis.Reprod Health. 2024 Jan 23;21(1):10. doi: 10.1186/s12978-024-01741-0. Reprod Health. 2024. PMID: 38263119 Free PMC article. Review.
-
Characteristics and quality of systematic reviews and meta-analyses of observational nutritional epidemiology: a cross-sectional study.Am J Clin Nutr. 2021 Jun 1;113(6):1578-1592. doi: 10.1093/ajcn/nqab002. Am J Clin Nutr. 2021. PMID: 33740039 Free PMC article.
-
Systematic reviews and meta-analyses of home telemonitoring interventions for patients with chronic diseases: a critical assessment of their methodological quality.J Med Internet Res. 2013 Jul 23;15(7):e150. doi: 10.2196/jmir.2770. J Med Internet Res. 2013. PMID: 23880072 Free PMC article. Review.
-
Bevacizumab Combined with Chemotherapy Improves Survival for Patients with Metastatic Colorectal Cancer: Evidence from Meta Analysis.PLoS One. 2016 Aug 31;11(8):e0161912. doi: 10.1371/journal.pone.0161912. eCollection 2016. PLoS One. 2016. PMID: 27579775 Free PMC article.
References
-
- Sidik K, Jonkman JN. Simple heterogeneity variance estimation for meta-analysis. JRSS Series C (Applied Statistics) 2005;54:367–384. doi: 10.1111/j.1467-9876.2005.00489.x. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

