Role of neutrophils in response to Bordetella pertussis infection in mice
- PMID: 19103765
- PMCID: PMC2643619
- DOI: 10.1128/IAI.01150-08
Role of neutrophils in response to Bordetella pertussis infection in mice
Abstract
Pertussis is an acute respiratory disease caused by the bacterium Bordetella pertussis, for which humans are the only known reservoir. During infection, B. pertussis releases several toxins, including pertussis toxin (PT) and adenylate cyclase toxin (ACT), which have both been shown to play roles in promoting bacterial growth during early infection in a mouse model. Furthermore, in vitro and in vivo studies suggest that PT and ACT affect neutrophil chemotaxis and/or function, thereby altering the innate immune response. In this study we depleted animals of neutrophils to investigate whether neutrophils play a protective role during B. pertussis infection in mice. In addition, by infection with toxin-deficient strains, we investigated whether neutrophils are the main _targets for PT and/or ACT activity in promoting bacterial growth. Surprisingly, we found no role for neutrophils during B. pertussis infection in naïve mice. However, in previously infected (immune) mice or in mice receiving immune serum, we observed a significant role for neutrophils during infection. Furthermore, in this immune mouse model our evidence indicates that neutrophils appear to be the main _target cells for ACT, but not for PT.
Figures
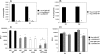
 , significantly different from the WT strain.
, significantly different from the WT strain.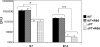
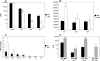
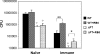
 , significantly different from the WT strain.
, significantly different from the WT strain.Similar articles
-
Pertussis toxin inhibits early chemokine production to delay neutrophil recruitment in response to Bordetella pertussis respiratory tract infection in mice.Infect Immun. 2008 Nov;76(11):5139-48. doi: 10.1128/IAI.00895-08. Epub 2008 Sep 2. Infect Immun. 2008. PMID: 18765723 Free PMC article.
-
Pertussis toxin and adenylate cyclase toxin provide a one-two punch for establishment of Bordetella pertussis infection of the respiratory tract.Infect Immun. 2005 May;73(5):2698-703. doi: 10.1128/IAI.73.5.2698-2703.2005. Infect Immun. 2005. PMID: 15845471 Free PMC article.
-
Review of the neutrophil response to Bordetella pertussis infection.Pathog Dis. 2015 Dec;73(9):ftv081. doi: 10.1093/femspd/ftv081. Epub 2015 Oct 2. Pathog Dis. 2015. PMID: 26432818 Free PMC article. Review.
-
Pertussis toxin exacerbates and prolongs airway inflammatory responses during Bordetella pertussis infection.Infect Immun. 2012 Dec;80(12):4317-32. doi: 10.1128/IAI.00808-12. Epub 2012 Oct 1. Infect Immun. 2012. PMID: 23027529 Free PMC article.
-
The adenylate cyclase toxin from Bordetella pertussis--a novel promising vehicle for antigen delivery to dendritic cells.Int J Med Microbiol. 2004 Apr;293(7-8):571-6. doi: 10.1078/1438-4221-00291. Int J Med Microbiol. 2004. PMID: 15149033 Review.
Cited by
-
Rediscovering Pertussis.Front Pediatr. 2016 Jun 8;4:52. doi: 10.3389/fped.2016.00052. eCollection 2016. Front Pediatr. 2016. PMID: 27376050 Free PMC article. Review.
-
Evaluation of Adenylate Cyclase Toxoid Antigen in Acellular Pertussis Vaccines by Using a Bordetella pertussis Challenge Model in Mice.Infect Immun. 2018 Sep 21;86(10):e00857-17. doi: 10.1128/IAI.00857-17. Print 2018 Oct. Infect Immun. 2018. PMID: 30012638 Free PMC article.
-
Blockade of the Adenylate Cyclase Toxin Synergizes with Opsonizing Antibodies to Protect Mice against Bordetella pertussis.mBio. 2022 Aug 30;13(4):e0152722. doi: 10.1128/mbio.01527-22. Epub 2022 Aug 3. mBio. 2022. PMID: 35920558 Free PMC article.
-
Live attenuated pertussis vaccine BPZE1 induces a broad antibody response in humans.J Clin Invest. 2020 May 1;130(5):2332-2346. doi: 10.1172/JCI135020. J Clin Invest. 2020. PMID: 31945015 Free PMC article. Clinical Trial.
-
Modeling Immune Evasion and Vaccine Limitations by _targeted Nasopharyngeal Bordetella pertussis Inoculation in Mice.Emerg Infect Dis. 2021 Aug;27(8):2107-2116. doi: 10.3201/eid2708.203566. Emerg Infect Dis. 2021. PMID: 34286682 Free PMC article.
References
-
- Addison, C. L., T. O. Daniel, M. D. Burdick, H. Liu, J. E. Ehlert, Y. Y. Xue, L. Buechi, A. Walz, A. Richmond, and R. M. Strieter. 2000. The CXC chemokine receptor 2, CXCR2, is the putative receptor for ELR+ CXC chemokine-induced angiogenic activity. J. Immunol. 1655269-5277. - PubMed
-
- Carbonetti, N. H., G. V. Artamonova, C. Andreasen, E. Dudley, R. M. Mays, and Z. E. Worthington. 2004. Suppression of serum antibody responses by pertussis toxin after respiratory tract colonization by Bordetella pertussis and identification of an immunodominant lipoprotein. Infect. Immun. 723350-3358. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

