The non-coding RNA gadd7 is a regulator of lipid-induced oxidative and endoplasmic reticulum stress
- PMID: 19150982
- PMCID: PMC2658040
- DOI: 10.1074/jbc.M806209200
The non-coding RNA gadd7 is a regulator of lipid-induced oxidative and endoplasmic reticulum stress
Abstract
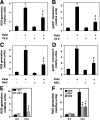
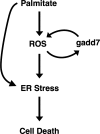
In obesity and diabetes, an imbalance in fatty acid uptake and fatty acid utilization leads to excess accumulation of lipid in non-adipose tissues. This lipid overload is associated with cellular dysfunction and cell death, which contribute to organ failure, a phenomenon termed lipotoxicity. To elucidate the molecular mechanism of lipid-mediated cell death, we generated and characterized a mutant Chinese hamster ovary cell line that is resistant to palmitate-induced cell death. In this mutant, random insertion of a retroviral promoter trap has disrupted the gene for the non-coding RNA, growth arrested DNA-damage inducible gene 7 (gadd7). Here we report that gadd7 is induced by lipotoxic stress in a reactive oxygen species (ROS)-dependent fashion and is necessary for both lipid- and general oxidative stress-mediated cell death. Depletion of gadd7 by mutagenesis or short hairpin RNA knockdown significantly reduces lipid and non-lipid induced ROS. Furthermore, depletion of gadd7 delays and diminishes ROS-induced endoplasmic reticulum stress. Together these data are the first to implicate a non-coding RNA in a feed-forward loop with oxidative stress and its induction of the endoplasmic reticulum stress response.
Figures


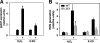
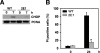
Similar articles
-
As a matter of fat.Cell Metab. 2009 Jul;10(1):9-12. doi: 10.1016/j.cmet.2009.03.011. Cell Metab. 2009. PMID: 19583949 Free PMC article. Review.
-
A critical role for eukaryotic elongation factor 1A-1 in lipotoxic cell death.Mol Biol Cell. 2006 Feb;17(2):770-8. doi: 10.1091/mbc.e05-08-0742. Epub 2005 Nov 30. Mol Biol Cell. 2006. PMID: 16319173 Free PMC article.
-
Disruption of endoplasmic reticulum structure and integrity in lipotoxic cell death.J Lipid Res. 2006 Dec;47(12):2726-37. doi: 10.1194/jlr.M600299-JLR200. Epub 2006 Sep 7. J Lipid Res. 2006. PMID: 16960261
-
Increased monocyte-derived reactive oxygen species in type 2 diabetes: role of endoplasmic reticulum stress.Exp Physiol. 2017 Feb 1;102(2):139-153. doi: 10.1113/EP085794. Epub 2017 Jan 10. Exp Physiol. 2017. PMID: 27859785 Free PMC article.
-
Oxidative stress and calcium dysregulation by palmitate in type 2 diabetes.Exp Mol Med. 2017 Feb 3;49(2):e291. doi: 10.1038/emm.2016.157. Exp Mol Med. 2017. PMID: 28154371 Free PMC article. Review.
Cited by
-
Melatonin restores zinc levels, activates the Keap1/Nrf2 pathway, and modulates endoplasmic reticular stress and HSP in rats with chronic hepatotoxicity.World J Gastrointest Pharmacol Ther. 2022 Mar 5;13(2):11-22. doi: 10.4292/wjgpt.v13.i2.11. World J Gastrointest Pharmacol Ther. 2022. PMID: 35433098 Free PMC article.
-
As a matter of fat.Cell Metab. 2009 Jul;10(1):9-12. doi: 10.1016/j.cmet.2009.03.011. Cell Metab. 2009. PMID: 19583949 Free PMC article. Review.
-
Creating and curing fatty hearts.Curr Opin Clin Nutr Metab Care. 2010 Mar;13(2):145-9. doi: 10.1097/MCO.0b013e3283357272. Curr Opin Clin Nutr Metab Care. 2010. PMID: 20010095 Free PMC article. Review.
-
Molecular mechanisms of diabetic cardiomyopathy.Diabetologia. 2014 Apr;57(4):660-71. doi: 10.1007/s00125-014-3171-6. Epub 2014 Jan 30. Diabetologia. 2014. PMID: 24477973 Free PMC article. Review.
-
MicroRNAs and Noncoding RNAs in Hepatic Lipid and Lipoprotein Metabolism: Potential Therapeutic _targets of Metabolic Disorders.Drug Dev Res. 2015 Sep;76(6):318-27. doi: 10.1002/ddr.21269. Epub 2015 Aug 19. Drug Dev Res. 2015. PMID: 26286650 Free PMC article. Review.
References
-
- Nollen, E. A., and Morimoto, R. I. (2002) J. Cell Sci. 115 2809–2816 - PubMed
-
- Zhang, K., and Kaufman, R. J. (2004) J. Biol. Chem. 279 25935–25938 - PubMed
-
- Unger, R. H. (2002) Annu. Rev. Med. 53 319–336 - PubMed
-
- Leichman, J. G., Aguilar, D., King, T. M., Vlada, A., Reyes, M., and Taegtmeyer, H. (2006) Am. J. Clin. Nutr. 84 336–341 - PubMed
-
- Sharma, S., Adrogue, J. V., Golfman, L., Uray, I., Lemm, J., Youker, K., Noon, G. P., Frazier, O. H., and Taegtmeyer, H. (2004) FASEB J. 18 1692–1700 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

