Coexpression of alpha 2A-adrenergic and delta-opioid receptors in substance P-containing terminals in rat dorsal horn
- PMID: 19180644
- PMCID: PMC2745955
- DOI: 10.1002/cne.21982
Coexpression of alpha 2A-adrenergic and delta-opioid receptors in substance P-containing terminals in rat dorsal horn
Erratum in
- J Comp Neurol. 2009 Jun 20;514(6):674
Abstract
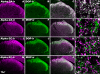
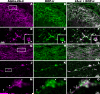
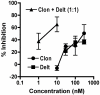
Agonists acting at alpha(2)-adrenergic and opioid receptors (alpha(2)ARs and ORs, respectively) inhibit pain transmission in the spinal cord. When coadministered, agonists activating these receptors interact in a synergistic manner. Although the existence of alpha(2)AR/OR synergy has been well characterized, its mechanism remains poorly understood. The formation of heterooligomers has been proposed as a molecular basis for interactions between neuronal G-protein-coupled receptors. The relevance of heterooligomer formation to spinal analgesic synergy requires demonstration of the expression of both receptors within the same neuron as well as the localization of both receptors in the same neuronal compartment. We used immunohistochemistry to investigate the spatial relationship between alpha(2)ARs and ORs in the rat spinal cord to determine whether coexpression could be demonstrated between these receptors. We observed extensive colocalization between alpha(2A)-adrenergic and delta-opioid receptors (DOP) on substance P (SP)-immunoreactive (-ir) varicosities in the superficial dorsal horn of the spinal cord and in peripheral nerve terminals in the skin. alpha(2A)AR- and DOP-ir elements were colocalized in subcellular structures of 0.5 mum or less in diameter in isolated nerve terminals. Furthermore, coincubation of isolated synaptosomes with alpha(2)AR and DOP agonists resulted in a greater-than-additive increase in the inhibition of K(+)-stimulated neuropeptide release. These findings suggest that coexpression of the synergistic receptor pair alpha(2A)AR-DOP on primary afferent nociceptive fibers may represent an anatomical substrate for analgesic synergy, perhaps as a result of protein-protein interactions such as heterooligomerization.
(c) 2009 Wiley-Liss, Inc.
Figures




Similar articles
-
Pharmacological profiles of alpha 2 adrenergic receptor agonists identified using genetically altered mice and isobolographic analysis.Pharmacol Ther. 2009 Aug;123(2):224-38. doi: 10.1016/j.pharmthera.2009.04.001. Epub 2009 Apr 23. Pharmacol Ther. 2009. PMID: 19393691 Free PMC article. Review.
-
Protein kinase C mediates the synergistic interaction between agonists acting at alpha2-adrenergic and delta-opioid receptors in spinal cord.J Neurosci. 2009 Oct 21;29(42):13264-73. doi: 10.1523/JNEUROSCI.1907-09.2009. J Neurosci. 2009. PMID: 19846714 Free PMC article.
-
Calcitonin receptor-like receptor and receptor activity modifying protein 1 in the rat dorsal horn: localization in glutamatergic presynaptic terminals containing opioids and adrenergic alpha2C receptors.Neuroscience. 2007 Aug 10;148(1):250-65. doi: 10.1016/j.neuroscience.2007.05.036. Epub 2007 Jul 5. Neuroscience. 2007. PMID: 17614212 Free PMC article.
-
Presynaptic localization of the carboxy-terminus epitopes of the mu opioid receptor splice variants MOR-1C and MOR-1D in the superficial laminae of the rat spinal cord.Neuroscience. 2001;106(4):833-42. doi: 10.1016/s0306-4522(01)00317-7. Neuroscience. 2001. PMID: 11682168
-
Feed-forward inhibition: a novel cellular mechanism for the analgesic effect of substance P.Mol Pain. 2005 Nov 18;1:34. doi: 10.1186/1744-8069-1-34. Mol Pain. 2005. PMID: 16297242 Free PMC article. Review.
Cited by
-
The delta opioid receptor tool box.Neuroscience. 2016 Dec 3;338:145-159. doi: 10.1016/j.neuroscience.2016.06.028. Epub 2016 Jun 24. Neuroscience. 2016. PMID: 27349452 Free PMC article. Review.
-
Recent advances on the δ opioid receptor: from trafficking to function.Br J Pharmacol. 2015 Jan;172(2):403-19. doi: 10.1111/bph.12706. Epub 2014 Jul 1. Br J Pharmacol. 2015. PMID: 24665909 Free PMC article. Review.
-
Pharmacological profiles of alpha 2 adrenergic receptor agonists identified using genetically altered mice and isobolographic analysis.Pharmacol Ther. 2009 Aug;123(2):224-38. doi: 10.1016/j.pharmthera.2009.04.001. Epub 2009 Apr 23. Pharmacol Ther. 2009. PMID: 19393691 Free PMC article. Review.
-
Activation of spinal mu- and delta-opioid receptors potently inhibits substance P release induced by peripheral noxious stimuli.J Neurosci. 2011 Sep 14;31(37):13068-77. doi: 10.1523/JNEUROSCI.1817-11.2011. J Neurosci. 2011. PMID: 21917790 Free PMC article.
-
Topical Treatments and Their Molecular/Cellular Mechanisms in Patients with Peripheral Neuropathic Pain-Narrative Review.Pharmaceutics. 2021 Mar 26;13(4):450. doi: 10.3390/pharmaceutics13040450. Pharmaceutics. 2021. PMID: 33810493 Free PMC article. Review.
References
-
- Aantaa R, Marjamaki A, Scheinin M. Molecular pharmacology of alpha 2-adrenoceptor subtypes. Ann Med. 1995;27(4):439–449. - PubMed
-
- Abbadie C, Pan YX, Pasternak GW. Differential distribution in rat brain of mu opioid receptor carboxy terminal splice variants MOR-1C-like and MOR-1-like immunoreactivity: evidence for region-specific processing. J Comp Neurol. 2000;419(2):244–256. - PubMed
-
- Aicher SA, Sharma S, Cheng PY, Liu-Chen LY, Pickel VM. Dual ultrastructural localization of mu-opiate receptors and substance p in the dorsal horn. Synapse. 2000;36(1):12–20. - PubMed
-
- Alguacil LF, Morales L. Alpha-2 Adrenoceptor Ligands and Opioid Drugs: Pharmacological Interactions of Therapeutic Interest. Current Neuropharmacology. 2004;2(4):343–352.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

