Synaptotagmin 2 couples mucin granule exocytosis to Ca2+ signaling from endoplasmic reticulum
- PMID: 19208631
- PMCID: PMC2665099
- DOI: 10.1074/jbc.M807849200
Synaptotagmin 2 couples mucin granule exocytosis to Ca2+ signaling from endoplasmic reticulum
Abstract
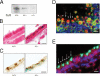
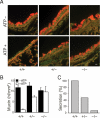
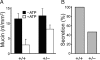
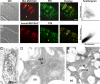
Synaptotagmin 2 (Syt2) functions as a low affinity, fast exocytic Ca(2+) sensor in neurons, where it is activated by Ca(2+) influx through voltage-gated channels. _targeted insertion of lacZ into the mouse syt2 locus reveals expression in mucin-secreting goblet cells of the airways. In these cells, rapid Ca(2+) entry from the extracellular medium does not contribute significantly to stimulated secretion (Davis, C. W., and Dickey, B. F. (2008) Annu. Rev. Physiol. 70, 487-512). Nonetheless, Syt2(-/-) mice show a severe defect in acute agonist-stimulated airway mucin secretion, and Syt2(+/-) mice show a partial defect. In contrast to Munc13-2(-/-) mice (Zhu, Y., Ehre, C., Abdullah, L. H., Sheehan, J. K., Roy, M., Evans, C. M., Dickey, B. F., and Davis, C. W. (2008) J. Physiol. (Lond.) 586, 1977-1992), Syt2(-/-) mice show no spontaneous mucin accumulation, consistent with the inhibitory action of Syt2 at resting cytoplasmic Ca(2+) in neurons. In human airway goblet cells, inositol trisphosphate receptors are found in rough endoplasmic reticulum that closely invests apical mucin granules, consistent with the known dependence of exocytic Ca(2+) signaling on intracellular stores in these cells. Hence, Syt2 can serve as an exocytic sensor for diverse Ca(2+) signaling systems, and its levels are limiting for stimulated secretory function in airway goblet cells.
Figures

Similar articles
-
Regulated mucin secretion from airway epithelial cells.Front Endocrinol (Lausanne). 2013 Sep 18;4:129. doi: 10.3389/fendo.2013.00129. Front Endocrinol (Lausanne). 2013. PMID: 24065956 Free PMC article. Review.
-
nPKCepsilon, a P2Y2-R downstream effector in regulated mucin secretion from airway goblet cells.Am J Physiol Cell Physiol. 2007 Nov;293(5):C1445-54. doi: 10.1152/ajpcell.00051.2007. Epub 2007 Aug 29. Am J Physiol Cell Physiol. 2007. PMID: 17728398
-
Barrier role of actin filaments in regulated mucin secretion from airway goblet cells.Am J Physiol Cell Physiol. 2005 Jan;288(1):C46-56. doi: 10.1152/ajpcell.00397.2004. Epub 2004 Sep 1. Am J Physiol Cell Physiol. 2005. PMID: 15342343
-
Synaptotagmin2 (Syt2) Drives Fast Release Redundantly with Syt1 at the Output Synapses of Parvalbumin-Expressing Inhibitory Neurons.J Neurosci. 2017 Apr 26;37(17):4604-4617. doi: 10.1523/JNEUROSCI.3736-16.2017. Epub 2017 Mar 31. J Neurosci. 2017. PMID: 28363983 Free PMC article.
-
Regulated airway goblet cell mucin secretion.Annu Rev Physiol. 2008;70:487-512. doi: 10.1146/annurev.physiol.70.113006.100638. Annu Rev Physiol. 2008. PMID: 17988208 Review.
Cited by
-
Identification of Hub Genes in Pediatric Medulloblastoma by Multiple-Microarray Analysis.J Mol Neurosci. 2020 Apr;70(4):522-531. doi: 10.1007/s12031-019-01451-4. Epub 2019 Dec 9. J Mol Neurosci. 2020. PMID: 31820345
-
Airway mucus function and dysfunction.N Engl J Med. 2010 Dec 2;363(23):2233-47. doi: 10.1056/NEJMra0910061. N Engl J Med. 2010. PMID: 21121836 Free PMC article. Review. No abstract available.
-
CFTR, mucins, and mucus obstruction in cystic fibrosis.Cold Spring Harb Perspect Med. 2012 Sep 1;2(9):a009589. doi: 10.1101/cshperspect.a009589. Cold Spring Harb Perspect Med. 2012. PMID: 22951447 Free PMC article. Review.
-
Regulated mucin secretion from airway epithelial cells.Front Endocrinol (Lausanne). 2013 Sep 18;4:129. doi: 10.3389/fendo.2013.00129. Front Endocrinol (Lausanne). 2013. PMID: 24065956 Free PMC article. Review.
-
Baseline Goblet Cell Mucin Secretion in the Airways Exceeds Stimulated Secretion over Extended Time Periods, and Is Sensitive to Shear Stress and Intracellular Mucin Stores.PLoS One. 2015 May 29;10(5):e0127267. doi: 10.1371/journal.pone.0127267. eCollection 2015. PLoS One. 2015. PMID: 26024524 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

