Pten deletion in adult neural stem/progenitor cells enhances constitutive neurogenesis
- PMID: 19211894
- PMCID: PMC2754186
- DOI: 10.1523/JNEUROSCI.3095-08.2009
Pten deletion in adult neural stem/progenitor cells enhances constitutive neurogenesis
Abstract
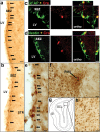
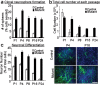
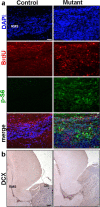
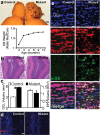
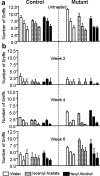
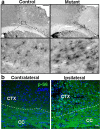
Here we show that conditional deletion of Pten in a subpopulation of adult neural stem cells in the subependymal zone (SEZ) leads to persistently enhanced neural stem cell self-renewal without sign of exhaustion. These Pten null SEZ-born neural stem cells and progenies can follow the endogenous migration, differentiation, and integration pathways and contribute to constitutive neurogenesis in the olfactory bulb. As a result, Pten deleted animals have increased olfactory bulb mass and enhanced olfactory function. Pten null cells in the olfactory bulb can establish normal connections with peripheral olfactory epithelium and help olfactory bulb recovery from acute damage. Following a focal stroke, Pten null progenitors give rise to greater numbers of neuroblasts that migrate to peri-infarct cortex. However, in contrast to the olfactory bulb, no significant long-term survival and integration can be observed, indicating that additional factors are necessary for long-term survival of newly born neurons after stroke. These data suggest that manipulating PTEN-controlled signaling pathways may be a useful step in facilitating endogenous neural stem/progenitor expansion for the treatment of disorders or lesions in regions associated with constitutive neurogenesis.
Figures

Similar articles
-
Pten deletion in adult hippocampal neural stem/progenitor cells causes cellular abnormalities and alters neurogenesis.J Neurosci. 2012 Apr 25;32(17):5880-90. doi: 10.1523/JNEUROSCI.5462-11.2012. J Neurosci. 2012. PMID: 22539849 Free PMC article.
-
Inducible activation of ERK5 MAP kinase enhances adult neurogenesis in the olfactory bulb and improves olfactory function.J Neurosci. 2015 May 20;35(20):7833-49. doi: 10.1523/JNEUROSCI.3745-14.2015. J Neurosci. 2015. PMID: 25995470 Free PMC article.
-
Involvement of Ngn2, Tbr and NeuroD proteins during postnatal olfactory bulb neurogenesis.Eur J Neurosci. 2009 Jan;29(2):232-43. doi: 10.1111/j.1460-9568.2008.06595.x. Eur J Neurosci. 2009. PMID: 19200230
-
[Neurogenesis in the adult brain. Functional consequences].J Soc Biol. 2002;196(1):67-76. J Soc Biol. 2002. PMID: 12134636 Review. French.
-
PTEN regulation of neural development and CNS stem cells.J Cell Biochem. 2003 Jan 1;88(1):24-8. doi: 10.1002/jcb.10312. J Cell Biochem. 2003. PMID: 12461771 Review.
Cited by
-
Excessive activation of mTOR in postnatally generated granule cells is sufficient to cause epilepsy.Neuron. 2012 Sep 20;75(6):1022-34. doi: 10.1016/j.neuron.2012.08.002. Neuron. 2012. PMID: 22998871 Free PMC article.
-
The alarmin interleukin-1α triggers secondary degeneration through reactive astrocytes and endothelium after spinal cord injury.Nat Commun. 2022 Oct 2;13(1):5786. doi: 10.1038/s41467-022-33463-x. Nat Commun. 2022. PMID: 36184639 Free PMC article.
-
Astrocytic glutamate transporter 1 (GLT1) deficient mice exhibit repetitive behaviors.Behav Brain Res. 2021 Jan 1;396:112906. doi: 10.1016/j.bbr.2020.112906. Epub 2020 Sep 17. Behav Brain Res. 2021. PMID: 32950606 Free PMC article.
-
Loss of fatty acid degradation by astrocytic mitochondria triggers neuroinflammation and neurodegeneration.Nat Metab. 2023 Mar;5(3):445-465. doi: 10.1038/s42255-023-00756-4. Epub 2023 Mar 23. Nat Metab. 2023. PMID: 36959514 Free PMC article.
-
mRNA Translation Is Dynamically Regulated to Instruct Stem Cell Fate.Front Mol Biosci. 2022 Mar 31;9:863885. doi: 10.3389/fmolb.2022.863885. eCollection 2022. Front Mol Biosci. 2022. PMID: 35433828 Free PMC article. Review.
References
-
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. - PubMed
-
- Backman SA, Stambolic V, Suzuki A, Haight J, Elia A, Pretorius J, Tsao MS, Shannon P, Bolon B, Ivy GO, Mak TW. Deletion of Pten in mouse brain causes seizures, ataxia and defects in soma size resembling Lhermitte-Duclos disease. Nat Genet. 2001;29:396–403. - PubMed
-
- Bhattacharyya A, Svendsen CN. Human neural stem cells: a new tool for studying cortical development in Down's syndrome. Genes Brain Behav. 2003;2:179–186. - PubMed
-
- Brunjes PC. Unilateral naris closure and olfactory system development. Brain Res Brain Res Rev. 1994;19:146–160. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS047386-05/NS/NINDS NIH HHS/United States
- NS047386/NS/NINDS NIH HHS/United States
- R01 NS047386/NS/NINDS NIH HHS/United States
- R01 NS047386-04/NS/NINDS NIH HHS/United States
- R01 MH065756/MH/NIMH NIH HHS/United States
- R01 NS047386-01/NS/NINDS NIH HHS/United States
- R01 NS047386-02/NS/NINDS NIH HHS/United States
- R01 NS053957-01A1/NS/NINDS NIH HHS/United States
- T32 CA09056/CA/NCI NIH HHS/United States
- R01 NS047386-03/NS/NINDS NIH HHS/United States
- T32 CA009056/CA/NCI NIH HHS/United States
- MH65756/MH/NIMH NIH HHS/United States
- R01 NS053957/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
