Source connectivity analysis with MEG and EEG
- PMID: 19235884
- PMCID: PMC6870611
- DOI: 10.1002/hbm.20745
Source connectivity analysis with MEG and EEG
Abstract
Interactions between functionally specialized brain regions are crucial for normal brain function. Magnetoencephalography (MEG) and electroencephalography (EEG) are techniques suited to capture these interactions, because they provide whole head measurements of brain activity in the millisecond range. More than one sensor picks up the activity of an underlying source. This field spread severely limits the utility of connectivity measures computed directly between sensor recordings. Consequentially, neuronal interactions should be studied on the level of the reconstructed sources. This article reviews several methods that have been applied to investigate interactions between brain regions in source space. We will mainly focus on the different measures used to quantify connectivity, and on the different strategies adopted to identify regions of interest. Despite various successful accounts of MEG and EEG source connectivity, caution with respect to the interpretation of the results is still warranted. This is due to the fact that effects of field spread can never be completely abolished in source space. However, in this very exciting and developing field of research this cautionary note should not discourage researchers from further investigation into the connectivity between neuronal sources.
(c) 2009 Wiley-Liss, Inc.
Figures
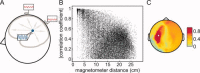
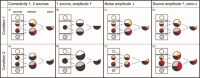
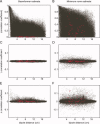
Similar articles
-
Hyperedge bundling: A practical solution to spurious interactions in MEG/EEG source connectivity analyses.Neuroimage. 2018 Jun;173:610-622. doi: 10.1016/j.neuroimage.2018.01.056. Epub 2018 Jan 31. Neuroimage. 2018. PMID: 29378318
-
Ghost interactions in MEG/EEG source space: A note of caution on inter-areal coupling measures.Neuroimage. 2018 Jun;173:632-643. doi: 10.1016/j.neuroimage.2018.02.032. Epub 2018 Feb 22. Neuroimage. 2018. PMID: 29477441
-
Phase shift invariant imaging of coherent sources (PSIICOS) from MEG data.Neuroimage. 2018 Dec;183:950-971. doi: 10.1016/j.neuroimage.2018.08.031. Epub 2018 Aug 22. Neuroimage. 2018. PMID: 30142449
-
Opportunities and methodological challenges in EEG and MEG resting state functional brain network research.Clin Neurophysiol. 2015 Aug;126(8):1468-81. doi: 10.1016/j.clinph.2014.11.018. Epub 2014 Nov 28. Clin Neurophysiol. 2015. PMID: 25511636 Review.
-
Magnetoencephalography for localizing and characterizing the epileptic focus.Handb Clin Neurol. 2019;160:203-214. doi: 10.1016/B978-0-444-64032-1.00013-8. Handb Clin Neurol. 2019. PMID: 31277848 Review.
Cited by
-
Spectral fingerprints of large-scale neuronal interactions.Nat Rev Neurosci. 2012 Jan 11;13(2):121-34. doi: 10.1038/nrn3137. Nat Rev Neurosci. 2012. PMID: 22233726 Review.
-
Source space analysis of event-related dynamic reorganization of brain networks.Comput Math Methods Med. 2012;2012:452503. doi: 10.1155/2012/452503. Epub 2012 Oct 11. Comput Math Methods Med. 2012. PMID: 23097678 Free PMC article.
-
VEP correlates of feedback in human cortex.PLoS One. 2012;7(12):e51791. doi: 10.1371/journal.pone.0051791. Epub 2012 Dec 12. PLoS One. 2012. PMID: 23251625 Free PMC article.
-
Modeling of Large-Scale Functional Brain Networks Based on Structural Connectivity from DTI: Comparison with EEG Derived Phase Coupling Networks and Evaluation of Alternative Methods along the Modeling Path.PLoS Comput Biol. 2016 Aug 9;12(8):e1005025. doi: 10.1371/journal.pcbi.1005025. eCollection 2016 Aug. PLoS Comput Biol. 2016. PMID: 27504629 Free PMC article.
-
Resting-state network disruption and APOE genotype in Alzheimer's disease: a lagged functional connectivity study.PLoS One. 2012;7(9):e46289. doi: 10.1371/journal.pone.0046289. Epub 2012 Sep 25. PLoS One. 2012. PMID: 23050006 Free PMC article.
References
-
- Astolfi L,Cincotti F,Mattia D,Salinari S,Babiloni C,Basilisco A,Rossini PM,Ding L,Ni Y,He B,Marciani MG,Babiloni F ( 2004): Estimation of the effective and functional human cortical connectivity with structural equation modeling and directed transfer function applied to high‐resolution EEG. Magn Reson Imaging 22: 1457–1470. - PubMed
-
- Astolfi L,Cincotti F,Mattia D,Babiloni C,Carducci F,Basilisco A,Rossini PM,Salinari S,Ding L,Ni Y,He B,Babiloni F ( 2005): Assessing cortical functional connectivity by linear inverse estimation and directed transfer function: simulations and application to real data. Clin Neurophysiol 116: 920–932. - PubMed
-
- Astolfi L,Cincotti F,Mattia D,De Vico Fallani F,Tocci A,Colosimo A,Salinari S,Marciani MG,Hesse W,Witte H,Ursino M,Zavaglia M,Babiloni F ( 2008): Tracking the time‐varying cortical connectivity patterns by adaptive multivariate estimators. IEEE Trans Biomed Eng 55: 902–913. - PubMed
-
- Babiloni F,Cincotti F,Babiloni C,Carducci F,Mattia D,Astolfi L,Basilisco A,Rossini PM,Ding L,Ni Y,Cheng J,Christine K,Sweeney J,He B ( 2005): Estimation of the cortical functional connectivity with the multimodal integration of high‐resolution EEG and fMRI data by directed transfer function. Neuroimage 24: 118–131. - PubMed
-
- Baccala LA,Sameshima K ( 2001): Partial directed coherence: A new concept in neural structure determination. Biol Cybern 84: 463–474. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

