PINK1-associated Parkinson's disease is caused by neuronal vulnerability to calcium-induced cell death
- PMID: 19285945
- PMCID: PMC2724101
- DOI: 10.1016/j.molcel.2009.02.013
PINK1-associated Parkinson's disease is caused by neuronal vulnerability to calcium-induced cell death
Abstract
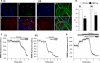
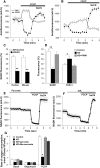
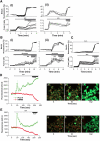
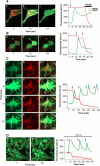
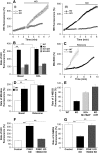
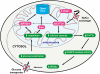
Mutations in PINK1 cause autosomal recessive Parkinson's disease. PINK1 is a mitochondrial kinase of unknown function. We investigated calcium homeostasis and mitochondrial function in PINK1-deficient mammalian neurons. We demonstrate physiologically that PINK1 regulates calcium efflux from the mitochondria via the mitochondrial Na(+)/Ca(2+) exchanger. PINK1 deficiency causes mitochondrial accumulation of calcium, resulting in mitochondrial calcium overload. We show that calcium overload stimulates reactive oxygen species (ROS) production via NADPH oxidase. ROS production inhibits the glucose transporter, reducing substrate delivery and causing impaired respiration. We demonstrate that impaired respiration may be restored by provision of mitochondrial complex I and II substrates. Taken together, reduced mitochondrial calcium capacity and increased ROS lower the threshold of opening of the mitochondrial permeability transition pore (mPTP) such that physiological calcium stimuli become sufficient to induce mPTP opening in PINK1-deficient cells. Our findings propose a mechanism by which PINK1 dysfunction renders neurons vulnerable to cell death.
Figures

Similar articles
-
Dopamine induced neurodegeneration in a PINK1 model of Parkinson's disease.PLoS One. 2012;7(5):e37564. doi: 10.1371/journal.pone.0037564. Epub 2012 May 25. PLoS One. 2012. PMID: 22662171 Free PMC article.
-
Increased mitochondrial calcium sensitivity and abnormal expression of innate immunity genes precede dopaminergic defects in Pink1-deficient mice.PLoS One. 2011 Jan 13;6(1):e16038. doi: 10.1371/journal.pone.0016038. PLoS One. 2011. PMID: 21249202 Free PMC article.
-
Regulation of mitochondrial permeability transition pore by PINK1.Mol Neurodegener. 2012 May 25;7:22. doi: 10.1186/1750-1326-7-22. Mol Neurodegener. 2012. PMID: 22630785 Free PMC article.
-
Mitochondrial Calcium Deregulation in the Mechanism of Beta-Amyloid and Tau Pathology.Cells. 2020 Sep 21;9(9):2135. doi: 10.3390/cells9092135. Cells. 2020. PMID: 32967303 Free PMC article. Review.
-
Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release.Physiol Rev. 2014 Jul;94(3):909-50. doi: 10.1152/physrev.00026.2013. Physiol Rev. 2014. PMID: 24987008 Free PMC article. Review.
Cited by
-
PINK1 contained in huMSC-derived exosomes prevents cardiomyocyte mitochondrial calcium overload in sepsis via recovery of mitochondrial Ca2+ efflux.Stem Cell Res Ther. 2021 May 6;12(1):269. doi: 10.1186/s13287-021-02325-6. Stem Cell Res Ther. 2021. PMID: 33957982 Free PMC article.
-
Mitochondrial protein dysfunction in pathogenesis of neurological diseases.Front Mol Neurosci. 2022 Sep 7;15:974480. doi: 10.3389/fnmol.2022.974480. eCollection 2022. Front Mol Neurosci. 2022. PMID: 36157077 Free PMC article. Review.
-
Mitochondria, calcium-dependent neuronal death and neurodegenerative disease.Pflugers Arch. 2012 Jul;464(1):111-21. doi: 10.1007/s00424-012-1112-0. Epub 2012 May 22. Pflugers Arch. 2012. PMID: 22615071 Free PMC article. Review.
-
The Links between Parkinson's Disease and Cancer.Biomedicines. 2020 Oct 14;8(10):416. doi: 10.3390/biomedicines8100416. Biomedicines. 2020. PMID: 33066407 Free PMC article. Review.
-
Loss of mitochondrial Ca2+ response and CaMKII/ERK activation by LRRK2R1441G mutation correlate with impaired depolarization-induced mitophagy.Cell Commun Signal. 2024 Oct 10;22(1):485. doi: 10.1186/s12964-024-01844-y. Cell Commun Signal. 2024. PMID: 39390438 Free PMC article.
References
-
- Abramov A.Y., Duchen M.R. Actions of ionomycin, 4-BrA23187 and a novel electrogenic Ca2+ ionophore on mitochondria in intact cells. Cell Calcium. 2003;33:101–112. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

