Mimitin - a novel cytokine-regulated mitochondrial protein
- PMID: 19331698
- PMCID: PMC2667391
- DOI: 10.1186/1471-2121-10-23
Mimitin - a novel cytokine-regulated mitochondrial protein
Abstract
Background: The product of a novel cytokine-responsive gene discovered by differential display analysis in our earlier studies on HepG2 cells was identified as mimitin - a small mitochondrial protein. Since proinflammatory cytokines are known to affect components of the respiratory chain in mitochondria, and mimitin was reported as a possible chaperone for assembly of mitochondrial complex I, we looked for the effects of modulation of mimitin expression and for mimitin-binding partners.
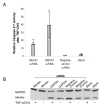
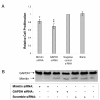
Results: By blocking mimitin expression in HepG2 cells by siRNA we found that mimitin has no direct influence on caspase 3/7 activities implicated in apoptosis. However, when apoptosis was induced by TNF and cycloheximide, and mimitin expression blocked, the activities of these caspases were significantly increased. This was accompanied by a slight decrease in proliferation of HepG2 cells. Our observations suggest that mimitin may be involved in the control of apoptosis indirectly, through another protein, or proteins. Using the yeast two-hybrid system and coimmunoprecipitation we found MAP1S among proteins interacting with mimitin. MAP1S is a recently identified member of the microtubule-associated protein family and has been shown to interact with NADH dehydrogenase I and cytochrome oxidase I. Moreover, it was implicated in the process of mitochondrial aggregation and nuclear genome destruction. The expression of mimitin is stimulated more than 1.6-fold by IL-1 and by IL-6, with the maximum level of mimitin observed after 18-24 h exposure to these cytokines. We also found that the cytokine-induced signal leading to stimulation of mimitin synthesis utilizes the MAP kinase pathway.
Conclusion: Mimitin is a mitochondrial protein upregulated by proinflammatory cytokines at the transcriptional and protein levels, with MAP kinases involved in IL-1-dependent induction. Mimitin interacts with a microtubular protein (MAP1S), and some changes of mimitin gene expression modulate activity of apoptotic caspases 3/7, suggesting that this protein may indirectly participate in apoptosis.
Figures






Similar articles
-
Effects of the novel mitochondrial protein mimitin in insulin-secreting cells.Biochem J. 2012 Aug 1;445(3):349-59. doi: 10.1042/BJ20111920. Biochem J. 2012. PMID: 22587331
-
A novel Myc-_target gene, mimitin, that is involved in cell proliferation of esophageal squamous cell carcinoma.J Biol Chem. 2005 May 20;280(20):19977-85. doi: 10.1074/jbc.M501231200. Epub 2005 Mar 17. J Biol Chem. 2005. PMID: 15774466
-
Interferon-gamma-induced sensitization of colon carcinomas to ZD9331 _targets caspases, downstream of Fas, independent of mitochondrial signaling and the inhibitor of apoptosis survivin.Clin Cancer Res. 2003 Dec 15;9(17):6504-15. Clin Cancer Res. 2003. PMID: 14695155
-
Involvement of smad2 and Erk/Akt cascade in TGF-β1-induced apoptosis in human gingival epithelial cells.Cytokine. 2015 Sep;75(1):165-73. doi: 10.1016/j.cyto.2015.03.011. Epub 2015 Apr 14. Cytokine. 2015. PMID: 25882870
-
The role of microtubule-associated protein 1S in SOCS3 regulation of IL-6 signaling.FEBS Lett. 2008 Dec 10;582(29):4015-22. doi: 10.1016/j.febslet.2008.10.055. Epub 2008 Nov 21. FEBS Lett. 2008. PMID: 19027008
Cited by
-
The mitochondrial disease associated protein Ndufaf2 is dispensable for Complex-1 assembly but critical for the regulation of oxidative stress.Neurobiol Dis. 2013 Oct;58:57-67. doi: 10.1016/j.nbd.2013.05.007. Epub 2013 May 20. Neurobiol Dis. 2013. PMID: 23702311 Free PMC article.
-
Effects triggered by platinum nanoparticles on primary keratinocytes.Int J Nanomedicine. 2013;8:3963-75. doi: 10.2147/IJN.S49612. Epub 2013 Oct 16. Int J Nanomedicine. 2013. PMID: 24204135 Free PMC article.
-
Identification of Potential Causal Genes for Neurodegenerative Diseases by Mitochondria-Related Genome-Wide Mendelian Randomization.Mol Neurobiol. 2024 Sep 30. doi: 10.1007/s12035-024-04528-3. Online ahead of print. Mol Neurobiol. 2024. PMID: 39347895
-
Ndufaf2, a protein in mitochondrial complex I, interacts in vivo with methionine sulfoxide reductases.Redox Rep. 2023 Dec;28(1):2168635. doi: 10.1080/13510002.2023.2168635. Redox Rep. 2023. PMID: 36738241 Free PMC article.
-
Quantitative proteomic analysis of mitochondria from human ovarian cancer cells and their paclitaxel-resistant sublines.Cancer Sci. 2015 Aug;106(8):1075-83. doi: 10.1111/cas.12710. Epub 2015 Jul 24. Cancer Sci. 2015. PMID: 26033570 Free PMC article.
References
-
- Koj A, Jura J. Complex analysis of genes involved in the inflammatory response: interleukin-1-induced differential transcriptome of cultured human hepatoma HepG2 cells. Acta Biochim Pol. 2003;50:573–582. - PubMed
-
- Wegrzyn P, Jura J, Kupiec T, Piekoszewski W, Władyka B, Zarebski A, Koj A. A search for genes modulated by interleukin-6 alone or with interleukin-1beta in HepG2 cells using differential display analysis. Biochim Biophys Acta. 2006;1762:319–328. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

