NEMO/IKKgamma regulates an early NF-kappaB-independent cell-death checkpoint during TNF signaling
- PMID: 19373245
- PMCID: PMC2728158
- DOI: 10.1038/cdd.2009.41
NEMO/IKKgamma regulates an early NF-kappaB-independent cell-death checkpoint during TNF signaling
Abstract
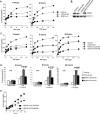
TNF receptor 1 (TNFR1) ligation can result in cell survival or cell death. What determines which of the two opposing responses is triggered is not fully understood. The current model suggests that it is the activation of the NF-kappaB pathway and its induction of prosurvival genes, or the lack thereof, which determines the outcome. NF-kappaB essential modifier (NEMO)/IkappaB kinase-gamma (IKKgamma)-deficient cells are highly sensitive to apoptosis, and as NEMO is essential for NF-kappaB activation, it has been assumed that this is due to the lack of NF-kappaB. This study demonstrates that this assumption was incorrect and that NEMO has another antiapoptotic function that is independent of its role in the NF-kappaB pathway. NEMO prevents receptor interacting protein-1 (RIP1) from engaging CASPASE-8 before NF-kappaB-mediated induction of antiapoptotic genes. Without NEMO, RIP1 associates with CASPASE-8 resulting in rapid tumor necrosis factor (TNF)-induced apoptosis. These results suggest that there are two cell-death checkpoints following TNF stimulation: an early transcription-independent checkpoint whereby NEMO restrains RIP1 from activating the caspase cascade, followed by a later checkpoint dependent on NF-kappaB-mediated transcription of prosurvival genes.
Figures

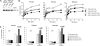
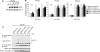

Similar articles
-
NEMO inhibits programmed necrosis in an NFκB-independent manner by restraining RIP1.PLoS One. 2012;7(7):e41238. doi: 10.1371/journal.pone.0041238. Epub 2012 Jul 26. PLoS One. 2012. PMID: 22848449 Free PMC article.
-
Protein Kinase-Mediated Decision Between the Life and Death.Adv Exp Med Biol. 2021;1275:1-33. doi: 10.1007/978-3-030-49844-3_1. Adv Exp Med Biol. 2021. PMID: 33539010
-
Inhibition of the NF-kappaB survival pathway via caspase-dependent cleavage of the IKK complex scaffold protein and NF-kappaB essential modulator NEMO.Cell Death Differ. 2008 Jan;15(1):152-60. doi: 10.1038/sj.cdd.4402240. Epub 2007 Oct 12. Cell Death Differ. 2008. PMID: 17932497
-
If the prophet does not come to the mountain: dynamics of signaling complexes in NF-kappaB activation.Mol Cell. 2006 May 19;22(4):433-6. doi: 10.1016/j.molcel.2006.05.002. Mol Cell. 2006. PMID: 16713572 Review.
-
The zinc finger domain of IKKγ (NEMO) protein in health and disease.J Cell Mol Med. 2010 Oct;14(10):2404-14. doi: 10.1111/j.1582-4934.2010.01054.x. J Cell Mol Med. 2010. PMID: 20345847 Free PMC article. Review.
Cited by
-
Chronicles of a death foretold: dual sequential cell death checkpoints in TNF signaling.Cell Cycle. 2010 Mar 15;9(6):1065-71. doi: 10.4161/cc.9.6.10982. Epub 2010 Mar 15. Cell Cycle. 2010. PMID: 20237426 Free PMC article. Review.
-
RIP1 comes back to life as a cell death regulator in TNFR1 signaling.FEBS J. 2011 Apr;278(6):877-87. doi: 10.1111/j.1742-4658.2011.08016.x. Epub 2011 Feb 8. FEBS J. 2011. PMID: 21232018 Free PMC article.
-
Ripoptocide - A Spark for Inflammation.Front Cell Dev Biol. 2019 Aug 13;7:163. doi: 10.3389/fcell.2019.00163. eCollection 2019. Front Cell Dev Biol. 2019. PMID: 31457011 Free PMC article. Review.
-
Regulation of a distinct activated RIPK1 intermediate bridging complex I and complex II in TNFα-mediated apoptosis.Proc Natl Acad Sci U S A. 2018 Jun 26;115(26):E5944-E5953. doi: 10.1073/pnas.1806973115. Epub 2018 Jun 11. Proc Natl Acad Sci U S A. 2018. PMID: 29891719 Free PMC article.
-
A novel pVHL-independent but NEMO-driven pathway in renal cancer promotes HIF stabilization.Oncogene. 2016 Jun 16;35(24):3125-38. doi: 10.1038/onc.2015.400. Epub 2015 Oct 26. Oncogene. 2016. PMID: 26500060
References
-
- Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol. 2003;3:745–56. - PubMed
-
- Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114:181–90. - PubMed
-
- Yamaoka S, Courtois G, Bessia C, Whiteside ST, Weil R, Agou F, et al. Complementation cloning of NEMO, a component of the IkappaB kinase complex essential for NF-kappaB activation. Cell. 1998;93:1231–40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

