Ku-0063794 is a specific inhibitor of the mammalian _target of rapamycin (mTOR)
- PMID: 19402821
- PMCID: PMC2708931
- DOI: 10.1042/BJ20090489
Ku-0063794 is a specific inhibitor of the mammalian _target of rapamycin (mTOR)
Abstract
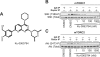
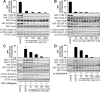
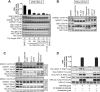
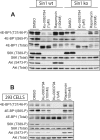
mTOR (mammalian _target of rapamycin) stimulates cell growth by phosphorylating and promoting activation of AGC (protein kinase A/protein kinase G/protein kinase C) family kinases such as Akt (protein kinase B), S6K (p70 ribosomal S6 kinase) and SGK (serum and glucocorticoid protein kinase). mTORC1 (mTOR complex-1) phosphorylates the hydrophobic motif of S6K, whereas mTORC2 phosphorylates the hydrophobic motif of Akt and SGK. In the present paper we describe the small molecule Ku-0063794, which inhibits both mTORC1 and mTORC2 with an IC50 of approximately 10 nM, but does not suppress the activity of 76 other protein kinases or seven lipid kinases, including Class 1 PI3Ks (phosphoinositide 3-kinases) at 1000-fold higher concentrations. Ku-0063794 is cell permeant, suppresses activation and hydrophobic motif phosphorylation of Akt, S6K and SGK, but not RSK (ribosomal S6 kinase), an AGC kinase not regulated by mTOR. Ku-0063794 also inhibited phosphorylation of the T-loop Thr308 residue of Akt phosphorylated by PDK1 (3-phosphoinositide-dependent protein kinase-1). We interpret this as implying phosphorylation of Ser473 promotes phosphorylation of Thr308 and/or induces a conformational change that protects Thr308 from dephosphorylation. In contrast, Ku-0063794 does not affect Thr308 phosphorylation in fibroblasts lacking essential mTORC2 subunits, suggesting that signalling processes have adapted to enable Thr308 phosphorylation to occur in the absence of Ser473 phosphorylation. We found that Ku-0063794 induced a much greater dephosphorylation of the mTORC1 substrate 4E-BP1 (eukaryotic initiation factor 4E-binding protein 1) than rapamycin, even in mTORC2-deficient cells, suggesting a form of mTOR distinct from mTORC1, or mTORC2 phosphorylates 4E-BP1. Ku-0063794 also suppressed cell growth and induced a G1-cell-cycle arrest. Our results indicate that Ku-0063794 will be useful in delineating the physiological roles of mTOR and may have utility in treatment of cancers in which this pathway is inappropriately activated.
Figures



Similar articles
-
mTOR complex 2 (mTORC2) controls hydrophobic motif phosphorylation and activation of serum- and glucocorticoid-induced protein kinase 1 (SGK1).Biochem J. 2008 Dec 15;416(3):375-85. doi: 10.1042/BJ20081668. Biochem J. 2008. PMID: 18925875
-
Distinct signaling mechanisms of mTORC1 and mTORC2 in glioblastoma multiforme: a tale of two complexes.Adv Biol Regul. 2015 Jan;57:64-74. doi: 10.1016/j.jbior.2014.09.004. Epub 2014 Sep 18. Adv Biol Regul. 2015. PMID: 25442674
-
Active-site inhibitors of mTOR _target rapamycin-resistant outputs of mTORC1 and mTORC2.PLoS Biol. 2009 Feb 10;7(2):e38. doi: 10.1371/journal.pbio.1000038. PLoS Biol. 2009. PMID: 19209957 Free PMC article.
-
Diverse signaling mechanisms of mTOR complexes: mTORC1 and mTORC2 in forming a formidable relationship.Adv Biol Regul. 2019 May;72:51-62. doi: 10.1016/j.jbior.2019.03.003. Epub 2019 Apr 11. Adv Biol Regul. 2019. PMID: 31010692 Review.
-
Discrete signaling mechanisms of mTORC1 and mTORC2: Connected yet apart in cellular and molecular aspects.Adv Biol Regul. 2017 May;64:39-48. doi: 10.1016/j.jbior.2016.12.001. Epub 2017 Jan 4. Adv Biol Regul. 2017. PMID: 28189457 Review.
Cited by
-
Potentiation of the Anticancer Effects by Combining Docetaxel with Ku-0063794 against Triple-Negative Breast Cancer Cells.Cancer Res Treat. 2022 Jan;54(1):157-173. doi: 10.4143/crt.2020.1063. Epub 2021 Apr 5. Cancer Res Treat. 2022. PMID: 33831291 Free PMC article.
-
Combined inhibition of PI3K-related DNA damage response kinases and mTORC1 induces apoptosis in MYC-driven B-cell lymphomas.Blood. 2013 Apr 11;121(15):2964-74. doi: 10.1182/blood-2012-08-446096. Epub 2013 Feb 12. Blood. 2013. PMID: 23403624 Free PMC article.
-
Inhibition of PI3K-Akt-mTOR signaling in glioblastoma by mTORC1/2 inhibitors.Methods Mol Biol. 2012;821:349-59. doi: 10.1007/978-1-61779-430-8_22. Methods Mol Biol. 2012. PMID: 22125077 Free PMC article.
-
Combination of ATP-competitive mammalian _target of rapamycin inhibitors with standard chemotherapy for colorectal cancer.Invest New Drugs. 2012 Dec;30(6):2219-25. doi: 10.1007/s10637-012-9793-y. Epub 2012 Jan 24. Invest New Drugs. 2012. PMID: 22270257
-
Methylene blue-induced neuronal protective mechanism against hypoxia-reoxygenation stress.Neuroscience. 2015 Aug 20;301:193-203. doi: 10.1016/j.neuroscience.2015.05.064. Epub 2015 Jun 3. Neuroscience. 2015. PMID: 26047733 Free PMC article.
References
-
- Wullschleger S., Loewith R., Hall M. N. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. - PubMed
-
- Sarbassov D. D., Ali S. M., Sabatini D. M. Growing roles for the mTOR pathway. Curr. Opin. Cell Biol. 2005;17:596–603. - PubMed
-
- Loewith R., Jacinto E., Wullschleger S., Lorberg A., Crespo J. L., Bonenfant D., Oppliger W., Jenoe P., Hall M. N. Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol. Cell. 2002;10:457–468. - PubMed
-
- Hara K., Maruki Y., Long X., Yoshino K., Oshiro N., Hidayat S., Tokunaga C., Avruch J., Yonezawa K. Raptor, a binding partner of _target of rapamycin (TOR), mediates TOR action. Cell. 2002;110:177–189. - PubMed
-
- Kim D. H., Sarbassov D. D., Ali S. M., King J. E., Latek R. R., Erdjument-Bromage H., Tempst P., Sabatini D. M. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110:163–175. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

