Dkk-3 is elevated in CSF and plasma of Alzheimer's disease patients
- PMID: 19457090
- PMCID: PMC4311140
- DOI: 10.1111/j.1471-4159.2009.06158.x
Dkk-3 is elevated in CSF and plasma of Alzheimer's disease patients
Abstract
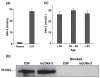
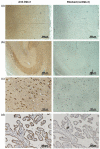
Biomarkers in CSF can offer improved diagnostic accuracy for Alzheimer's disease (AD). The present study investigated whether the glycoprotein and putative tumor suppressor Dickkopf homolog 3 (Dkk-3) is secreted into CSF and evaluated its applicability as a diagnostic marker for AD. Using our highly specific immunoenzymometric assay, Dkk-3 levels were measured in plasma and/or CSF of patients suffering from depression, mild cognitive impairment (MCI), or AD and compared with healthy subjects. Dkk-3 identity was verified by western blot and matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry (MS)/MS. High concentrations of Dkk-3 were detected in CSF compared with plasma (28.2 +/- 1.3 vs. 1.22 +/- 0.04 nmol/L, respectively). Consistently Dkk-3 expression was demonstrated in neurons of the cortex and epithelial cells of the choroid plexus, the major source of CSF. Significantly increased Dkk-3 levels in plasma and CSF were observed for AD patients compared with healthy subjects but not patients suffering from MCI or depression. In summary, our data indicate that elevated Dkk-3 levels are specifically associated with AD and might serve as a potential non-invasive AD biomarker in plasma.
Figures

Similar articles
-
Macrophage colony-stimulating factor (M-CSF) in plasma and CSF of patients with mild cognitive impairment and Alzheimer's disease.Curr Alzheimer Res. 2010 Aug;7(5):409-14. doi: 10.2174/156720510791383813. Curr Alzheimer Res. 2010. PMID: 20455868
-
Growth factors and cytokines/chemokines as surrogate biomarkers in cerebrospinal fluid and blood for diagnosing Alzheimer's disease and mild cognitive impairment.Exp Gerontol. 2010 Jan;45(1):41-6. doi: 10.1016/j.exger.2009.10.011. Epub 2009 Oct 22. Exp Gerontol. 2010. PMID: 19853649 Review.
-
Does serum uric acid act as a modulator of cerebrospinal fluid Alzheimer's disease biomarker related cognitive decline?Eur J Neurol. 2016 May;23(5):948-57. doi: 10.1111/ene.12969. Epub 2016 Feb 24. Eur J Neurol. 2016. PMID: 26917248
-
A New Serum Biomarker Set to Detect Mild Cognitive Impairment and Alzheimer's Disease by Peptidome Technology.J Alzheimers Dis. 2020;73(1):217-227. doi: 10.3233/JAD-191016. J Alzheimers Dis. 2020. PMID: 31771070 Free PMC article.
-
Update on the biomarker core of the Alzheimer's Disease Neuroimaging Initiative subjects.Alzheimers Dement. 2010 May;6(3):230-8. doi: 10.1016/j.jalz.2010.03.008. Alzheimers Dement. 2010. PMID: 20451871 Free PMC article. Review.
Cited by
-
Differential expression of Wnts after spinal cord contusion injury in adult rats.PLoS One. 2011;6(11):e27000. doi: 10.1371/journal.pone.0027000. Epub 2011 Nov 2. PLoS One. 2011. PMID: 22073235 Free PMC article.
-
Blood-based biomarkers of Alzheimer's disease: challenging but feasible.Biomark Med. 2010 Feb;4(1):65-79. doi: 10.2217/bmm.09.84. Biomark Med. 2010. PMID: 20387303 Free PMC article. Review.
-
Elevations in Serum Dickkopf-1 and Disease Progression in Community-Dwelling Older Adults With Mild Cognitive Impairment and Mild-to-Moderate Alzheimer's Disease.Front Aging Neurosci. 2019 Oct 15;11:278. doi: 10.3389/fnagi.2019.00278. eCollection 2019. Front Aging Neurosci. 2019. PMID: 31680933 Free PMC article.
-
Dickkopf-related protein 3 promotes pathogenic stromal remodeling in benign prostatic hyperplasia and prostate cancer.Prostate. 2013 Sep;73(13):1441-52. doi: 10.1002/pros.22691. Epub 2013 Jun 14. Prostate. 2013. PMID: 23765731 Free PMC article.
-
Cognitive impairment in long-living adults: a genome-wide association study, polygenic risk score model and molecular modeling of the APOE protein.Front Aging Neurosci. 2023 Oct 26;15:1273825. doi: 10.3389/fnagi.2023.1273825. eCollection 2023. Front Aging Neurosci. 2023. PMID: 37953886 Free PMC article.
References
-
- Bibl M, Mollenhauer B, Esselmann H, et al. Cerebrospinal fluid neurochemical phenotypes in vascular dementias: original data and mini-review. Dement. Geriatr. Cogn. Disord. 2008;25:256–265. - PubMed
-
- Blasko I, Lederer W, Oberbauer H, Walch T, Kemmler G, Hinterhuber H, Marksteiner J, Humpel C. Measurement of thirteen biological markers in CSF of patients with Alzheimer’s disease and other dementias. Dement. Geriatr. Cogn. Disord. 2006;21:9–15. - PubMed
-
- Blennow K. CSF biomarkers for mild cognitive impairment. J. Intern. Med. 2004;256:224–234. - PubMed
-
- Blennow K. CSF biomarkers for Alzheimer’s disease: use in early diagnosis and evaluation of drug treatment. Expert Rev. Mol. Diagn. 2005;5:661–672. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

