Unique roles of DosT and DosS in DosR regulon induction and Mycobacterium tuberculosis dormancy
- PMID: 19487478
- PMCID: PMC2715697
- DOI: 10.1128/IAI.01449-08
Unique roles of DosT and DosS in DosR regulon induction and Mycobacterium tuberculosis dormancy
Abstract
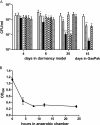
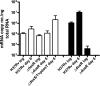
In Mycobacterium tuberculosis, the sensor kinases DosT and DosS activate the transcriptional regulator DosR, resulting in the induction of the DosR regulon, which is important for anaerobic survival and perhaps latent infection. The individual and collective roles of these sensors have been postulated biochemically, but their roles in vivo have remained unclear. This work demonstrates distinct and additive roles for each sensor during anaerobic dormancy. Both sensors are necessary for wild-type levels of DosR regulon induction, and concomitantly, full induction of the regulon is required for wild-type anaerobic survival. In the anaerobic model, DosT plays an early role, responding to hypoxia. DosT then induces the regulon and with it DosS, which sustains and further induces the regulon. DosT then loses its functionality as oxygen becomes limited, and DosS alone maintains induction of the genes from that point forward. Thus, M. tuberculosis has evolved a system whereby it responds to hypoxic conditions in a stepwise fashion as it enters an anaerobic state.
Figures
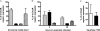
Similar articles
-
Mycobacterium tuberculosis DosS is a redox sensor and DosT is a hypoxia sensor.Proc Natl Acad Sci U S A. 2007 Jul 10;104(28):11568-73. doi: 10.1073/pnas.0705054104. Epub 2007 Jul 3. Proc Natl Acad Sci U S A. 2007. PMID: 17609369 Free PMC article.
-
DosS Is required for the complete virulence of mycobacterium tuberculosis in mice with classical granulomatous lesions.Am J Respir Cell Mol Biol. 2015 Jun;52(6):708-16. doi: 10.1165/rcmb.2014-0230OC. Am J Respir Cell Mol Biol. 2015. PMID: 25322074 Free PMC article.
-
Strains of the East Asian (W/Beijing) lineage of Mycobacterium tuberculosis are DosS/DosT-DosR two-component regulatory system natural mutants.J Bacteriol. 2010 Apr;192(8):2228-38. doi: 10.1128/JB.01597-09. Epub 2010 Feb 12. J Bacteriol. 2010. PMID: 20154135 Free PMC article.
-
How Mycobacterium tuberculosis goes to sleep: the dormancy survival regulator DosR a decade later.Future Microbiol. 2012 Apr;7(4):513-8. doi: 10.2217/fmb.12.14. Future Microbiol. 2012. PMID: 22439727 Review.
-
Immunogenic potential of latency associated antigens against Mycobacterium tuberculosis.Vaccine. 2014 Feb 3;32(6):712-6. doi: 10.1016/j.vaccine.2013.11.065. Epub 2013 Dec 2. Vaccine. 2014. PMID: 24300592 Review.
Cited by
-
Individual Mycobacterium tuberculosis universal stress protein homologues are dispensable in vitro.Tuberculosis (Edinb). 2010 Jul;90(4):236-44. doi: 10.1016/j.tube.2010.03.013. Epub 2010 Jun 11. Tuberculosis (Edinb). 2010. PMID: 20541977 Free PMC article.
-
The dormancy regulator DosR controls ribosome stability in hypoxic mycobacteria.J Biol Chem. 2012 Jul 6;287(28):24053-63. doi: 10.1074/jbc.M112.364851. Epub 2012 Apr 27. J Biol Chem. 2012. PMID: 22544737 Free PMC article.
-
Discovery of selective menaquinone biosynthesis inhibitors against Mycobacterium tuberculosis.J Med Chem. 2012 Apr 26;55(8):3739-55. doi: 10.1021/jm201608g. Epub 2012 Apr 6. J Med Chem. 2012. PMID: 22449052 Free PMC article.
-
Role of Oxidative Stress in the Pathology and Management of Human Tuberculosis.Oxid Med Cell Longev. 2018 Oct 11;2018:7695364. doi: 10.1155/2018/7695364. eCollection 2018. Oxid Med Cell Longev. 2018. PMID: 30405878 Free PMC article. Review.
-
Defining the Transcriptional and Post-transcriptional Landscapes of Mycobacterium smegmatis in Aerobic Growth and Hypoxia.Front Microbiol. 2019 Mar 26;10:591. doi: 10.3389/fmicb.2019.00591. eCollection 2019. Front Microbiol. 2019. PMID: 30984135 Free PMC article.
References
-
- Anonymous. 1953. Report of panel discussion on survival and revival of tubercle bacilli in healed tuberculous lesions. Am. Rev. Tuberc. 68477-495. - PubMed
-
- Bardarov, S., S. Bardarov, Jr., M. S. Pavelka, Jr., V. Sambandamurthy, M. Larsen, J. Tufariello, J. Chan, G. Hatfull, and W. R. Jacobs, Jr. 2002. Specialized transduction: an efficient method for generating marked and unmarked _targeted gene disruptions in Mycobacterium tuberculosis, M. bovis BCG and M. smegmatis. Microbiology 1483007-3017. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

