Reliable neuromodulation from circuits with variable underlying structure
- PMID: 19553211
- PMCID: PMC2701344
- DOI: 10.1073/pnas.0905614106
Reliable neuromodulation from circuits with variable underlying structure
Abstract
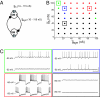
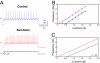
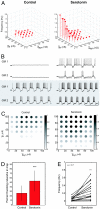
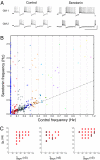
Recent work argues that similar network performance can result from highly variable sets of network parameters, raising the question of whether neuromodulation can be reliable across individuals with networks with different sets of synaptic strengths and intrinsic membrane conductances. To address this question, we used the dynamic clamp to construct 2-cell reciprocally inhibitory networks from gastric mill (GM) neurons of the crab stomatogastric ganglion. When the strength of the artificial inhibitory synapses (g(syn)) and the conductance of an artificial I(h) (g(h)) were varied with the dynamic clamp, a variety of network behaviors resulted, including regions of stable alternating bursting. Maps of network output as a function of g(syn) and g(h) were constructed in normal saline and again in the presence of serotonin or oxotremorine. Both serotonin and oxotremorine depolarize and excite isolated individual GM neurons, but by different cellular mechanisms. Serotonin and oxotremorine each increased the size of the parameter regions that supported alternating bursting, and, on average, increased burst frequency. Nonetheless, in both cases some parameter sets within the sample space deviated from the mean population response and decreased in frequency. These data provide insight into why pharmacological treatments that work in most individuals can generate anomalous actions in a few individuals, and they have implications for understanding the evolution of nervous systems.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Prinz AA, Bucher D, Marder E. Similar network activity from disparate circuit parameters. Nat Neurosci. 2004;7:1345–1352. - PubMed
-
- Schulz DJ, Goaillard JM, Marder E. Variable channel expression in identified single and electrically coupled neurons in different animals. Nat Neurosci. 2006;9:356–362. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

