Differential matrix rigidity response in breast cancer cell lines correlates with the tissue tropism
- PMID: 19626122
- PMCID: PMC2709918
- DOI: 10.1371/journal.pone.0006361
Differential matrix rigidity response in breast cancer cell lines correlates with the tissue tropism
Abstract
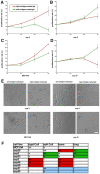
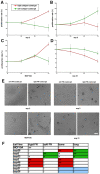
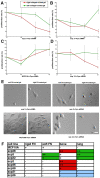
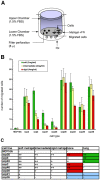
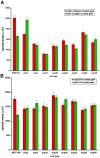
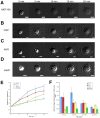
Metastasis to a variety of distant organs, such as lung, brain, bone, and liver, is a leading cause of mortality in the breast cancer patients. The tissue tropism of breast cancer metastasis has been recognized and studied extensively, but the cellular processes underlying this phenomenon, remain elusive. Modern technologies have enabled the discovery of a number of the genetic factors determining tissue tropism of malignant cells. However, the effect of these genetic differences on the cell motility and invasiveness is poorly understood. Here, we report that cellular responses to the mechanical rigidity of the extracellular matrix correlate with the rigidity of the _target tissue. We tested a series of single cell populations isolated from MDA-MB-231 breast cancer cell line in a variety of assays where the extracellular matrix rigidity was varied to mimic the environment that these cells might encounter in vivo. There was increased proliferation and migration through the matrices of rigidities corresponding to the native rigidities of the organs where metastasis was observed. We were able to abolish the differential matrix rigidity response by knocking down Fyn kinase, which was previously identified as a critical component of the FN rigidity response pathway in healthy cells. This result suggests possible molecular mechanisms of the rigidity response in the malignant cells, indicating potential candidates for therapeutic interventions.
Conflict of interest statement
Figures
Similar articles
-
Actomyosin tension as a determinant of metastatic cancer mechanical tropism.Phys Biol. 2015 Feb 23;12(2):026001. doi: 10.1088/1478-3975/12/2/026001. Phys Biol. 2015. PMID: 25706686
-
New tricks against an old foe: molecular dissection of metastasis tissue tropism in breast cancer.Breast Dis. 2006-2007;26:129-38. doi: 10.3233/bd-2007-26111. Breast Dis. 2006. PMID: 17473371 Review.
-
Suppression of distant pulmonary metastasis of MDA-MB 435 human breast carcinoma established in mammary fat pads of nude mice by retroviral-mediated TIMP-2 gene transfer.J Gene Med. 2005 Feb;7(2):145-57. doi: 10.1002/jgm.645. J Gene Med. 2005. PMID: 15546163
-
Effect of matrix rigidity on organ-specific capture of tumor cells by flow.Cell Mol Biol (Noisy-le-grand). 2017 Apr 29;63(4):10-15. doi: 10.14715/cmb/2017.63.4.2. Cell Mol Biol (Noisy-le-grand). 2017. PMID: 28478797
-
Materials and extracellular matrix rigidity highlighted in tissue damages and diseases: Implication for biomaterials design and therapeutic _targets.Bioact Mater. 2022 Jun 16;20:381-403. doi: 10.1016/j.bioactmat.2022.06.003. eCollection 2023 Feb. Bioact Mater. 2022. PMID: 35784640 Free PMC article. Review.
Cited by
-
A multiwell platform for studying stiffness-dependent cell biology.PLoS One. 2011;6(5):e19929. doi: 10.1371/journal.pone.0019929. Epub 2011 May 27. PLoS One. 2011. PMID: 21637769 Free PMC article.
-
A synthetic matrix with independently tunable biochemistry and mechanical properties to study epithelial morphogenesis and EMT in a lung adenocarcinoma model.Cancer Res. 2012 Nov 15;72(22):6013-23. doi: 10.1158/0008-5472.CAN-12-0895. Epub 2012 Sep 4. Cancer Res. 2012. PMID: 22952217 Free PMC article.
-
Dynamically Coupled Residues within the SH2 Domain of FYN Are Key to Unlocking Its Activity.Structure. 2016 Nov 1;24(11):1947-1959. doi: 10.1016/j.str.2016.08.016. Epub 2016 Sep 29. Structure. 2016. PMID: 27692963 Free PMC article.
-
Forcing form and function: biomechanical regulation of tumor evolution.Trends Cell Biol. 2011 Jan;21(1):47-56. doi: 10.1016/j.tcb.2010.08.015. Epub 2010 Oct 1. Trends Cell Biol. 2011. PMID: 20870407 Free PMC article. Review.
-
Bioelectric Dysregulation in Cancer Initiation, Promotion, and Progression.Front Oncol. 2022 Mar 14;12:846917. doi: 10.3389/fonc.2022.846917. eCollection 2022. Front Oncol. 2022. PMID: 35359398 Free PMC article. Review.
References
-
- Wittekind C, Neid M. Cancer invasion and metastasis. Oncology. 2005;69:14–16. - PubMed
-
- Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 1989;8:98–101. - PubMed
-
- Hart IR, Fidler IJ. Role of organ selectivity in the determination of metastatic patterns of B16 melanoma. Cancer Res. 1980;40:2281–2287. - PubMed
-
- Weigelt B, Hu Z, He X, Livasy C, Carey LA, et al. Molecular portraits and 70-gene prognosis signature are preserved throughout the metastatic process of breast cancer. Cancer Res. 2005;65:9155–9158. - PubMed
-
- Kirschmann DA, Seftor EA, Nieva DR, Mariano EA, Hendrix MJ. Differentially expressed genes associated with the metastatic phenotype in breast cancer. Breast Cancer Res Treat. 1999;55:127–136. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

