Amyloid-beta(1-42) fibrillar precursors are optimal for inducing tumor necrosis factor-alpha production in the THP-1 human monocytic cell line
- PMID: 19694428
- PMCID: PMC2749082
- DOI: 10.1021/bi9003777
Amyloid-beta(1-42) fibrillar precursors are optimal for inducing tumor necrosis factor-alpha production in the THP-1 human monocytic cell line
Abstract
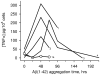
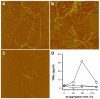
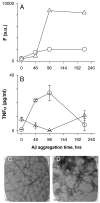
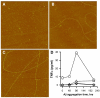
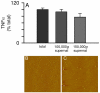
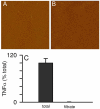
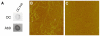
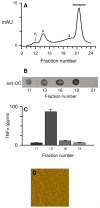
Pathological studies have determined that fibrillar forms of amyloid-beta protein (Abeta) comprise the characteristic neuritic plaques in Alzheimer's disease (AD). These studies have also revealed significant inflammatory markers such as activated microglia and cytokines surrounding the plaques. Although the plaques are a hallmark of AD, they are only part of an array of Abeta aggregate morphologies observed in vivo. Interestingly, not all of these Abeta deposits provoke an inflammatory response. Since structural polymorphism is a prominent feature of Abeta aggregation both in vitro and in vivo, we sought to clarify which Abeta morphology or aggregation species induces the strongest proinflammatory response using human THP-1 monocytes as a model system. An aliquot of freshly reconstituted Abeta(1-42) in sterile water (100 microM, pH 3.6) did not effectively stimulate the cells at a final Abeta concentration of 15 microM. However, quiescent incubation of the peptide at 4 degrees C for 48-96 h greatly enhanced its ability to induce tumor necrosis factor-alpha (TNFalpha) production, the level of which surprisingly declined upon further aggregation. Imaging of the Abeta(1-42) aggregation solutions with atomic force microscopy indicated that the best cellular response coincided with the appearance of fibrillar structures, yet conditions that accelerated or increased the level of Abeta(1-42) fibril formation such as peptide concentration, temperature, or reconstitution in NaOH/PBS at pH 7.4 diminished its ability to stimulate the cells. Finally, depletion of the Abeta(1-42) solution with an antibody that recognizes fibrillar oligomers dramatically weakened the ability to induce TNFalpha production, and size-exclusion separation of the Abeta(1-42) solution provided further characterization of an aggregated species with proinflammatory activity. The findings suggested that an intermediate stage Abeta(1-42) fibrillar precursor is optimal for inducing a proinflammatory response in THP-1 monocytes.
Figures
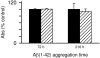
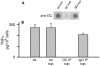
Similar articles
-
Ginger extract inhibits beta-amyloid peptide-induced cytokine and chemokine expression in cultured THP-1 monocytes.J Altern Complement Med. 2004 Dec;10(6):1009-13. doi: 10.1089/acm.2004.10.1009. J Altern Complement Med. 2004. PMID: 15673995
-
beta-Amyloid stimulation of microglia and monocytes results in TNFalpha-dependent expression of inducible nitric oxide synthase and neuronal apoptosis.J Neurosci. 2001 Feb 15;21(4):1179-88. doi: 10.1523/JNEUROSCI.21-04-01179.2001. J Neurosci. 2001. PMID: 11160388 Free PMC article.
-
Oligomeric amyloid-beta(1-42) induces THP-1 human monocyte adhesion and maturation.Brain Res. 2009 Feb 13;1254:109-19. doi: 10.1016/j.brainres.2008.11.093. Epub 2008 Dec 10. Brain Res. 2009. PMID: 19101527
-
Physicochemical characteristics of soluble oligomeric Abeta and their pathologic role in Alzheimer's disease.Neurol Res. 2005 Dec;27(8):869-81. doi: 10.1179/016164105X49436. Neurol Res. 2005. PMID: 16354549 Review.
-
Alzheimer's disease.Subcell Biochem. 2012;65:329-52. doi: 10.1007/978-94-007-5416-4_14. Subcell Biochem. 2012. PMID: 23225010 Review.
Cited by
-
Isolated amyloid-β(1-42) protofibrils, but not isolated fibrils, are robust stimulators of microglia.ACS Chem Neurosci. 2012 Apr 18;3(4):302-11. doi: 10.1021/cn2001238. Epub 2012 Jan 9. ACS Chem Neurosci. 2012. PMID: 22860196 Free PMC article.
-
Stability of early-stage amyloid-β(1-42) aggregation species.Biochim Biophys Acta. 2013 Jan;1834(1):65-70. doi: 10.1016/j.bbapap.2012.08.017. Epub 2012 Aug 25. Biochim Biophys Acta. 2013. PMID: 22944394 Free PMC article.
-
Loss of Direct Vascular Contact to Astrocytes in the Hippocampus as an Initial Event in Alzheimer's Disease. Evidence from Patients, In Vivo and In Vitro Experimental Models.Mol Neurobiol. 2024 Aug;61(8):5142-5160. doi: 10.1007/s12035-023-03897-5. Epub 2024 Jan 3. Mol Neurobiol. 2024. PMID: 38172288
-
Selective acetyl- and butyrylcholinesterase inhibitors reduce amyloid-β ex vivo activation of peripheral chemo-cytokines from Alzheimer's disease subjects: exploring the cholinergic anti-inflammatory pathway.Curr Alzheimer Res. 2014;11(6):608-22. doi: 10.2174/1567205010666131212113218. Curr Alzheimer Res. 2014. PMID: 24359497 Free PMC article.
-
The Aβ oligomer hypothesis for synapse failure and memory loss in Alzheimer's disease.Neurobiol Learn Mem. 2011 Nov;96(4):529-43. doi: 10.1016/j.nlm.2011.08.003. Epub 2011 Sep 6. Neurobiol Learn Mem. 2011. PMID: 21914486 Free PMC article. Review.
References
-
- Selkoe DJ. Cell biology of protein misfolding: The examples of Alzheimer’s and Parkinson’s diseases. Nat Cell Biol. 2004;6:1054–1061. - PubMed
-
- Selkoe DJ. The cell biology of β-amyloid precursor protein and presenilin in Alzheimer’s disease. Trends Cell Biol. 1998;8:447–453. - PubMed
-
- McGeer PL, Itagaki S, Tago H, McGeer EG. Reactive microglia in patients with senile dementia of the Alzheimer type are positive for the histocompatibility glycoprotein HLA-DR. Neurosci. Lett. 1987;79:195–200. - PubMed
-
- Dickson DW, Lee SC, Mattiace LA, Yen SHC, Brosnan C. Microglia and cytokines in neurological disease, with special reference to AIDS and Alzheimer disease. Glia. 1993;7:75–83. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

