Cellular and behavioral effects of cranial irradiation of the subventricular zone in adult mice
- PMID: 19753118
- PMCID: PMC2737283
- DOI: 10.1371/journal.pone.0007017
Cellular and behavioral effects of cranial irradiation of the subventricular zone in adult mice
Abstract
Background: In mammals, new neurons are added to the olfactory bulb (OB) throughout life. Most of these new neurons, granule and periglomerular cells originate from the subventricular zone (SVZ) lining the lateral ventricles and migrate via the rostral migratory stream toward the OB. Thousands of new neurons appear each day, but the function of this ongoing neurogenesis remains unclear.
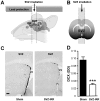
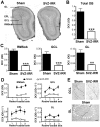
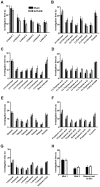
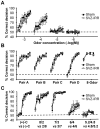
Methodology/principal findings: In this study, we irradiated adult mice to impair constitutive OB neurogenesis, and explored the functional impacts of this irradiation on the sense of smell. We found that focal irradiation of the SVZ greatly decreased the rate of production of new OB neurons, leaving other brain areas intact. This effect persisted for up to seven months after exposure to 15 Gray. Despite this robust impairment, the thresholds for detecting pure odorant molecules and short-term olfactory memory were not affected by irradiation. Similarly, the ability to distinguish between odorant molecules and the odorant-guided social behavior of irradiated mice were not affected by the decrease in the number of new neurons. Only long-term olfactory memory was found to be sensitive to SVZ irradiation.
Conclusion/significance: These findings suggest that the continuous production of adult-generated neurons is involved in consolidating or restituting long-lasting olfactory traces.
Conflict of interest statement
Figures
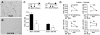

Similar articles
-
Age-related impairment of olfactory bulb neurogenesis in the Ts65Dn mouse model of Down syndrome.Exp Neurol. 2014 Jan;251:1-11. doi: 10.1016/j.expneurol.2013.10.018. Epub 2013 Nov 2. Exp Neurol. 2014. PMID: 24192151
-
Enriched odor exposure increases the number of newborn neurons in the adult olfactory bulb and improves odor memory.J Neurosci. 2002 Apr 1;22(7):2679-89. doi: 10.1523/JNEUROSCI.22-07-02679.2002. J Neurosci. 2002. PMID: 11923433 Free PMC article.
-
Hard-diet feeding recovers neurogenesis in the subventricular zone and olfactory functions of mice impaired by soft-diet feeding.PLoS One. 2014 May 9;9(5):e97309. doi: 10.1371/journal.pone.0097309. eCollection 2014. PLoS One. 2014. PMID: 24817277 Free PMC article.
-
The functional significance of newly born neurons integrated into olfactory bulb circuits.Front Neurosci. 2014 May 26;8:121. doi: 10.3389/fnins.2014.00121. eCollection 2014. Front Neurosci. 2014. PMID: 24904263 Free PMC article. Review.
-
Origin and function of olfactory bulb interneuron diversity.Trends Neurosci. 2008 Aug;31(8):392-400. doi: 10.1016/j.tins.2008.05.006. Epub 2008 Jul 5. Trends Neurosci. 2008. PMID: 18603310 Free PMC article. Review.
Cited by
-
Olfactory neurogenesis plays different parts at successive stages of life, implications for mental health.Front Neural Circuits. 2024 Aug 8;18:1467203. doi: 10.3389/fncir.2024.1467203. eCollection 2024. Front Neural Circuits. 2024. PMID: 39175668 Free PMC article. Review.
-
Single-cell RNA sequencing of aging neural progenitors reveals loss of excitatory neuron potential and a population with transcriptional immune response.Front Neurosci. 2024 Aug 9;18:1400963. doi: 10.3389/fnins.2024.1400963. eCollection 2024. Front Neurosci. 2024. PMID: 39184324 Free PMC article.
-
Excitable Axonal Domains Adapt to Sensory Deprivation in the Olfactory System.J Neurosci. 2022 Feb 23;42(8):1491-1509. doi: 10.1523/JNEUROSCI.0305-21.2021. Epub 2022 Jan 12. J Neurosci. 2022. PMID: 35022219 Free PMC article.
-
Olfactory learning promotes input-specific synaptic plasticity in adult-born neurons.Proc Natl Acad Sci U S A. 2014 Sep 23;111(38):13984-9. doi: 10.1073/pnas.1404991111. Epub 2014 Sep 4. Proc Natl Acad Sci U S A. 2014. PMID: 25189772 Free PMC article.
-
The Role of Adult-Born Neurons in the Constantly Changing Olfactory Bulb Network.Neural Plast. 2016;2016:1614329. doi: 10.1155/2016/1614329. Epub 2015 Dec 29. Neural Plast. 2016. PMID: 26839709 Free PMC article. Review.
References
-
- Dietrich J, Monje M, Wefel J, Meyers C. Clinical patterns and biological correlates of cognitive dysfunction associated with cancer therapy. Oncologist. 2008;13:1285–95. - PubMed
-
- Steinbach S, Hummel T, Böhner C, Berktold S, Hundt W, et al. Qualitative and quantitative assessment of taste and smell changes in patients undergoing chemotherapy for breast cancer or gynecologic malignancies. J Clin Oncol. 2009;27:1899–905. - PubMed
-
- Altman J, Das GD. Post-natal origin of microneurones in the rat brain. Nature. 1965;207:953–956. - PubMed
-
- Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;32:645–660. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

