Global effects of inactivation of the pyruvate kinase gene in the Mycobacterium tuberculosis complex
- PMID: 19820096
- PMCID: PMC2786599
- DOI: 10.1128/JB.00619-09
Global effects of inactivation of the pyruvate kinase gene in the Mycobacterium tuberculosis complex
Abstract
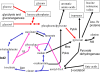
To better understand the global effects of "natural" lesions in genes involved in the pyruvate metabolism in Mycobacterium bovis, null mutations were made in the Mycobacterium tuberculosis H37Rv ald and pykA genes to mimic the M. bovis situation. Like M. bovis, the M. tuberculosis DeltapykA mutant yielded dysgonic colonies on solid medium lacking pyruvate, whereas colony morphology was eugonic on pyruvate-containing medium. Global effects of the loss of the pykA gene, possibly underlying colony morphology, were investigated by using proteomics on cultures grown in the same conditions. The levels of Icd2 increased and those of Icl and PckA decreased in the DeltapykA knockout. Proteomics suggested that the synthesis of enzymes involved in fatty acid and lipid biosynthesis were decreased, whereas those involved in beta-oxidation were increased in the M. tuberculosis DeltapykA mutant, as confirmed by direct assays for these activities. Thus, the loss of pykA from M. tuberculosis results in fatty acids being used principally for energy production, in contrast to the situation in the host when carbon from fatty acids is conserved through the glyoxylate cycle and gluconeogenesis; when an active pykA gene was introduced into M. bovis, the opposite effects occurred. Proteins involved in oxidative stress-AhpC, KatG, and SodA-showed increased synthesis in the DeltapykA mutant, and iron-regulated proteins were also affected. Ald levels were decreased in the DeltapykA knockout, explaining why an M. tuberculosis DeltapykA Deltaald double mutant showed little additional phenotypic effect. Overall, these data show that the loss of the pykA gene has powerful, global effects on proteins associated with central metabolism.
Figures
Similar articles
-
Central Role of Pyruvate Kinase in Carbon Co-catabolism of Mycobacterium tuberculosis.J Biol Chem. 2016 Mar 25;291(13):7060-9. doi: 10.1074/jbc.M115.707430. Epub 2016 Feb 8. J Biol Chem. 2016. PMID: 26858255 Free PMC article.
-
The pyruvate requirement of some members of the Mycobacterium tuberculosis complex is due to an inactive pyruvate kinase: implications for in vivo growth.Mol Microbiol. 2005 Apr;56(1):163-74. doi: 10.1111/j.1365-2958.2005.04524.x. Mol Microbiol. 2005. PMID: 15773987
-
ald of Mycobacterium tuberculosis encodes both the alanine dehydrogenase and the putative glycine dehydrogenase.J Bacteriol. 2012 Mar;194(5):1045-54. doi: 10.1128/JB.05914-11. Epub 2011 Dec 30. J Bacteriol. 2012. PMID: 22210765 Free PMC article.
-
Alanine dehydrogenases in mycobacteria.J Microbiol. 2019 Feb;57(2):81-92. doi: 10.1007/s12275-019-8543-7. Epub 2019 Jan 31. J Microbiol. 2019. PMID: 30706339 Review.
-
What Can Proteomics Tell Us about Tuberculosis?J Microbiol Biotechnol. 2015 Aug;25(8):1181-94. doi: 10.4014/jmb.1502.02008. J Microbiol Biotechnol. 2015. PMID: 25737117 Review.
Cited by
-
Central Role of Pyruvate Kinase in Carbon Co-catabolism of Mycobacterium tuberculosis.J Biol Chem. 2016 Mar 25;291(13):7060-9. doi: 10.1074/jbc.M115.707430. Epub 2016 Feb 8. J Biol Chem. 2016. PMID: 26858255 Free PMC article.
-
Biochemical Characterization of Isoniazid-resistant Mycobacterium tuberculosis: Can the Analysis of Clonal Strains Reveal Novel _targetable Pathways?Mol Cell Proteomics. 2018 Sep;17(9):1685-1701. doi: 10.1074/mcp.RA118.000821. Epub 2018 May 29. Mol Cell Proteomics. 2018. PMID: 29844232 Free PMC article.
-
Understanding the role of PknJ in Mycobacterium tuberculosis: biochemical characterization and identification of novel substrate pyruvate kinase A.PLoS One. 2010 May 24;5(5):e10772. doi: 10.1371/journal.pone.0010772. PLoS One. 2010. PMID: 20520732 Free PMC article.
-
PPE37 Is Essential for Mycobacterium tuberculosis Heme-Iron Acquisition (HIA), and a Defective PPE37 in Mycobacterium bovis BCG Prevents HIA.Infect Immun. 2019 Jan 24;87(2):e00540-18. doi: 10.1128/IAI.00540-18. Print 2019 Feb. Infect Immun. 2019. PMID: 30455201 Free PMC article.
-
Exploring virulence in Mycobacterium bovis: clues from comparative genomics and perspectives for the future.Ir Vet J. 2023 Sep 28;76(Suppl 1):26. doi: 10.1186/s13620-023-00257-6. Ir Vet J. 2023. PMID: 37770951 Free PMC article. Review.
References
-
- Besra, G. S. 1998. Preparation of cell-wall fractions from mycobacteria, p. 91-107. In T. Parish and N. G. Stoker (ed.), Mycobacteria protocols, vol. 101. Humana Press, Inc., Totowa, NJ. - PubMed
-
- Brosch, R., S. V. Gordon, M. Marmiesse, P. Brodin, C. Buchrieser, K. Eiglmeier, T. Garnier, C. Gutierrez, G. Hewinson, K. Kremer, L. M. Parsons, A. S. Pym, S. Samper, D. van Soolingen, and S. T. Cole. 2002. A new evolutionary scenario for the Mycobacterium tuberculosis complex. Proc. Natl. Acad. Sci. USA 99:3684-3689. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

