Autophagy regulates adipose mass and differentiation in mice
- PMID: 19855132
- PMCID: PMC2769174
- DOI: 10.1172/JCI39228
Autophagy regulates adipose mass and differentiation in mice
Abstract
The relative balance between the quantity of white and brown adipose tissue can profoundly affect lipid storage and whole-body energy homeostasis. However, the mechanisms regulating the formation, expansion, and interconversion of these 2 distinct types of fat remain unknown. Recently, the lysosomal degradative pathway of macroautophagy has been identified as a regulator of cellular differentiation, suggesting that autophagy may modulate this process in adipocytes. The function of autophagy in adipose differentiation was therefore examined in the current study by genetic inhibition of the critical macroautophagy gene autophagy-related 7 (Atg7). Knockdown of Atg7 in 3T3-L1 preadipocytes inhibited lipid accumulation and decreased protein levels of adipocyte differentiation factors. Knockdown of Atg5 or pharmacological inhibition of autophagy or lysosome function also had similar effects. An adipocyte-specific mouse knockout of Atg7 generated lean mice with decreased white adipose mass and enhanced insulin sensitivity. White adipose tissue in knockout mice had increased features of brown adipocytes, which, along with an increase in normal brown adipose tissue, led to an elevated rate of fatty acid, beta-oxidation, and a lean body mass. Autophagy therefore functions to regulate body lipid accumulation by controlling adipocyte differentiation and determining the balance between white and brown fat.
Figures
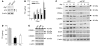

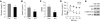

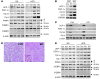
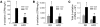
Similar articles
-
Adipose-specific deletion of autophagy-related gene 7 (atg7) in mice reveals a role in adipogenesis.Proc Natl Acad Sci U S A. 2009 Nov 24;106(47):19860-5. doi: 10.1073/pnas.0906048106. Epub 2009 Nov 12. Proc Natl Acad Sci U S A. 2009. PMID: 19910529 Free PMC article.
-
The Effect of FOXC2-AS1 on White Adipocyte Browning and the Possible Regulatory Mechanism.Front Endocrinol (Lausanne). 2020 Oct 29;11:565483. doi: 10.3389/fendo.2020.565483. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 33193083 Free PMC article.
-
miR-155 regulates differentiation of brown and beige adipocytes via a bistable circuit.Nat Commun. 2013;4:1769. doi: 10.1038/ncomms2742. Nat Commun. 2013. PMID: 23612310 Free PMC article.
-
UCP1 protein: The molecular hub of adipose organ plasticity.Biochimie. 2017 Mar;134:71-76. doi: 10.1016/j.biochi.2016.09.008. Epub 2016 Sep 10. Biochimie. 2017. PMID: 27622583 Review.
-
White, brown and pink adipocytes: the extraordinary plasticity of the adipose organ.Eur J Endocrinol. 2014 Apr 10;170(5):R159-71. doi: 10.1530/EJE-13-0945. Print 2014 May. Eur J Endocrinol. 2014. PMID: 24468979 Review.
Cited by
-
Involvement of TGF-β and Autophagy Pathways in Pathogenesis of Diabetes: A Comprehensive Review on Biological and Pharmacological Insights.Front Pharmacol. 2020 Sep 15;11:498758. doi: 10.3389/fphar.2020.498758. eCollection 2020. Front Pharmacol. 2020. PMID: 33041786 Free PMC article. Review.
-
Novel perspectives on autophagy-oxidative stress-inflammation axis in the orchestration of adipogenesis.Front Endocrinol (Lausanne). 2024 Jun 24;15:1404697. doi: 10.3389/fendo.2024.1404697. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 38982993 Free PMC article. Review.
-
Suppression of autophagy by FIP200 deletion leads to osteopenia in mice through the inhibition of osteoblast terminal differentiation.J Bone Miner Res. 2013 Nov;28(11):2414-30. doi: 10.1002/jbmr.1971. J Bone Miner Res. 2013. PMID: 23633228 Free PMC article.
-
The PRKAA1/AMPKα1 pathway triggers autophagy during CSF1-induced human monocyte differentiation and is a potential _target in CMML.Autophagy. 2015;11(7):1114-29. doi: 10.1080/15548627.2015.1034406. Autophagy. 2015. PMID: 26029847 Free PMC article.
-
Epidemiology and risk factors for avascular necrosis in childhood systemic lupus erythematosus in a Taiwanese population.Sci Rep. 2020 Sep 23;10(1):15563. doi: 10.1038/s41598-020-71923-w. Sci Rep. 2020. PMID: 32968109 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- AG031782/AG/NIA NIH HHS/United States
- DK020451/DK/NIDDK NIH HHS/United States
- DK020541/DK/NIDDK NIH HHS/United States
- R37 AG021904/AG/NIA NIH HHS/United States
- R01 DK047208/DK/NIDDK NIH HHS/United States
- DK061498/DK/NIDDK NIH HHS/United States
- P30 DK020541/DK/NIDDK NIH HHS/United States
- P60 DK020541/DK/NIDDK NIH HHS/United States
- R01 DK061498/DK/NIDDK NIH HHS/United States
- K01 DK087776/DK/NIDDK NIH HHS/United States
- R01 AG021904/AG/NIA NIH HHS/United States
- DK047208/DK/NIDDK NIH HHS/United States
- P01 AG031782/AG/NIA NIH HHS/United States
- AG021904/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

