Lin-Sca-1+CD49fhigh stem/progenitors are tumor-initiating cells in the Pten-null prostate cancer model
- PMID: 19887604
- PMCID: PMC2783355
- DOI: 10.1158/0008-5472.CAN-08-4673
Lin-Sca-1+CD49fhigh stem/progenitors are tumor-initiating cells in the Pten-null prostate cancer model
Abstract
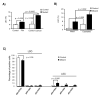
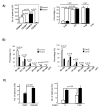
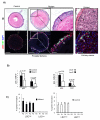
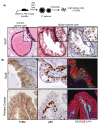
We have shown previously that Pten deletion leads to the expansion of subset of prostate cancer cells positive for CK5 and p63. Although this subpopulation may be involved in tumor initiation or progression, studies to date have not functionally validated this hypothesis. Using in vitro sphere-forming assay and in vivo prostate reconstitution assay, we show here the presence of a tumor-initiating subpopulation in the Pten prostate cancer mouse model. Specifically, we show that the Lin(-)Sca-1(+)CD49f(high) (LSC) subpopulation overlaps with CK5(+);p63(+) cells and is significantly increased during prostate cancer initiation and progression and after castration. Mutant spheres mimic the structural organization of the epithelial compartment in the Pten-null primary tumor. Sorted LSC cells from either Pten-null spheres or primary tumors are able to regenerate prostate epithelial structure with cancerous morphology, closely mimicking that of primary cancers. Therefore, the LSC subpopulation is capable of initiating a cancerous phenotype that recapitulates the pathology seen in the primary lesions of the Pten mutant prostate model.
Figures
Similar articles
-
A rare castration-resistant progenitor cell population is highly enriched in Pten-null prostate tumours.J Pathol. 2017 Sep;243(1):51-64. doi: 10.1002/path.4924. Epub 2017 Jul 28. J Pathol. 2017. PMID: 28603917
-
Identification of CD166 as a surface marker for enriching prostate stem/progenitor and cancer initiating cells.PLoS One. 2012;7(8):e42564. doi: 10.1371/journal.pone.0042564. Epub 2012 Aug 3. PLoS One. 2012. PMID: 22880034 Free PMC article.
-
Pten deletion leads to the expansion of a prostatic stem/progenitor cell subpopulation and tumor initiation.Proc Natl Acad Sci U S A. 2006 Jan 31;103(5):1480-5. doi: 10.1073/pnas.0510652103. Epub 2006 Jan 23. Proc Natl Acad Sci U S A. 2006. PMID: 16432235 Free PMC article.
-
Linneg Sca-1high CD49fhigh prostate cancer cells derived from the Hi-Myc mouse model are tumor-initiating cells with basal-epithelial characteristics and differentiation potential in vitro and in vivo.Onco_target. 2016 May 3;7(18):25194-207. doi: 10.18632/onco_target.7535. Onco_target. 2016. PMID: 26910370 Free PMC article.
-
Characterizing the contribution of stem/progenitor cells to tumorigenesis in the Pten-/-TP53-/- prostate cancer model.Stem Cells. 2010 Dec;28(12):2129-40. doi: 10.1002/stem.538. Stem Cells. 2010. PMID: 20936707 Free PMC article.
Cited by
-
CXCR4 expression in prostate cancer progenitor cells.PLoS One. 2012;7(2):e31226. doi: 10.1371/journal.pone.0031226. Epub 2012 Feb 16. PLoS One. 2012. PMID: 22359577 Free PMC article.
-
JNK and PTEN cooperatively control the development of invasive adenocarcinoma of the prostate.Proc Natl Acad Sci U S A. 2012 Jul 24;109(30):12046-51. doi: 10.1073/pnas.1209660109. Epub 2012 Jul 2. Proc Natl Acad Sci U S A. 2012. PMID: 22753496 Free PMC article.
-
The helix-loop-helix transcriptional regulator Id4 is required for terminal differentiation of luminal epithelial cells in the prostate.Oncoscience. 2021 Mar 24;8:14-30. doi: 10.18632/oncoscience.524. eCollection 2021. Oncoscience. 2021. PMID: 33884281 Free PMC article.
-
New insights into prostate cancer stem cells.Cell Cycle. 2013 Feb 15;12(4):579-86. doi: 10.4161/cc.23721. Epub 2013 Jan 31. Cell Cycle. 2013. PMID: 23370446 Free PMC article. Review.
-
Epigenetic regulation of CpG promoter methylation in invasive prostate cancer cells.Mol Cancer. 2010 Oct 7;9:267. doi: 10.1186/1476-4598-9-267. Mol Cancer. 2010. PMID: 20929579 Free PMC article.
References
-
- Gronberg H. Prostate cancer epidemiology. Lancet. 2003;361:859–64. - PubMed
-
- English HF, Santen RJ, Isaacs JT. Response of glandular versus basal rat ventral prostatic epithelial cells to androgen withdrawal and replacement. Prostate. 1987;11:229–42. - PubMed
-
- Isaacs J. U.S. Depart Health Hum Services; Washington, DC: 1985.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

