A neutralizing human monoclonal antibody protects against lethal disease in a new ferret model of acute nipah virus infection
- PMID: 19888339
- PMCID: PMC2765826
- DOI: 10.1371/journal.ppat.1000642
A neutralizing human monoclonal antibody protects against lethal disease in a new ferret model of acute nipah virus infection
Abstract
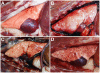
Nipah virus is a broadly tropic and highly pathogenic zoonotic paramyxovirus in the genus Henipavirus whose natural reservoirs are several species of Pteropus fruit bats. Nipah virus has repeatedly caused outbreaks over the past decade associated with a severe and often fatal disease in humans and animals. Here, a new ferret model of Nipah virus pathogenesis is described where both respiratory and neurological disease are present in infected animals. Severe disease occurs with viral doses as low as 500 TCID(50) within 6 to 10 days following infection. The underlying pathology seen in the ferret closely resembles that seen in Nipah virus infected humans, characterized as a widespread multisystemic vasculitis, with virus replicating in highly vascular tissues including lung, spleen and brain, with recoverable virus from a variety of tissues. Using this ferret model a cross-reactive neutralizing human monoclonal antibody, m102.4, _targeting the henipavirus G glycoprotein was evaluated in vivo as a potential therapeutic agent. All ferrets that received m102.4 ten hours following a high dose oral-nasal Nipah virus challenge were protected from disease while all controls died. This study is the first successful post-exposure passive antibody therapy for Nipah virus using a human monoclonal antibody.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Therapeutic treatment of Nipah virus infection in nonhuman primates with a neutralizing human monoclonal antibody.Sci Transl Med. 2014 Jun 25;6(242):242ra82. doi: 10.1126/scitranslmed.3008929. Sci Transl Med. 2014. PMID: 24964990 Free PMC article.
-
A Cross-Reactive Humanized Monoclonal Antibody _targeting Fusion Glycoprotein Function Protects Ferrets Against Lethal Nipah Virus and Hendra Virus Infection.J Infect Dis. 2020 May 11;221(Suppl 4):S471-S479. doi: 10.1093/infdis/jiz515. J Infect Dis. 2020. PMID: 31686101 Free PMC article.
-
Vaccination of ferrets with a recombinant G glycoprotein subunit vaccine provides protection against Nipah virus disease for over 12 months.Virol J. 2013 Jul 16;10:237. doi: 10.1186/1743-422X-10-237. Virol J. 2013. PMID: 23867060 Free PMC article.
-
Immunization strategies against henipaviruses.Curr Top Microbiol Immunol. 2012;359:197-223. doi: 10.1007/82_2012_213. Curr Top Microbiol Immunol. 2012. PMID: 22481140 Free PMC article. Review.
-
Henipavirus pathogenesis and antiviral approaches.Expert Rev Anti Infect Ther. 2015 Mar;13(3):343-54. doi: 10.1586/14787210.2015.1001838. Epub 2015 Jan 29. Expert Rev Anti Infect Ther. 2015. PMID: 25634624 Review.
Cited by
-
Antiviral Activity, Pharmacoinformatics, Molecular Docking, and Dynamics Studies of Azadirachta indica Against Nipah Virus by _targeting Envelope Glycoprotein: Emerging Strategies for Developing Antiviral Treatment.Bioinform Biol Insights. 2024 Jul 27;18:11779322241264145. doi: 10.1177/11779322241264145. eCollection 2024. Bioinform Biol Insights. 2024. PMID: 39072258 Free PMC article.
-
Henipavirus Encephalitis: Recent Developments and Advances.Brain Pathol. 2015 Sep;25(5):605-13. doi: 10.1111/bpa.12278. Brain Pathol. 2015. PMID: 26276024 Free PMC article. Review.
-
Henipavirus receptor usage and tropism.Curr Top Microbiol Immunol. 2012;359:59-78. doi: 10.1007/82_2012_222. Curr Top Microbiol Immunol. 2012. PMID: 22695915 Free PMC article. Review.
-
Nipah virus: epidemiology, pathology, immunobiology and advances in diagnosis, vaccine designing and control strategies - a comprehensive review.Vet Q. 2019 Dec;39(1):26-55. doi: 10.1080/01652176.2019.1580827. Vet Q. 2019. PMID: 31006350 Free PMC article. Review.
-
A single-dose ChAdOx1-vectored vaccine provides complete protection against Nipah Bangladesh and Malaysia in Syrian golden hamsters.PLoS Negl Trop Dis. 2019 Jun 6;13(6):e0007462. doi: 10.1371/journal.pntd.0007462. eCollection 2019 Jun. PLoS Negl Trop Dis. 2019. PMID: 31170144 Free PMC article.
References
-
- Bishop KA, Broder CC. Hendra and Nipah: Lethal Zoonotic Paramyxoviruses. In: Scheld WM, Hammer SM, Hughes JM, editors. Emerging Infections. Washington, D.C.: American Society for Microbiology; 2008. pp. 155–187.
-
- Hayman DT, Suu-Ire R, Breed AC, McEachern JA, Wang L, et al. Evidence of henipavirus infection in West African fruit bats. PLoS ONE. 2008;3:e2739. doi: 10.1371/journal.pone.0002739. - DOI - PMC - PubMed
-
- Anonymous. Hendra Virus, Human, Equine - Australia (07): (Queensland). Pro-MED (International Society for Infectious Diseases) (August 21, 2008, archive no. 20080821.2606). 2008. Available at www.promedmail.org.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

