Matrix crosslinking forces tumor progression by enhancing integrin signaling
- PMID: 19931152
- PMCID: PMC2788004
- DOI: 10.1016/j.cell.2009.10.027
Matrix crosslinking forces tumor progression by enhancing integrin signaling
Abstract
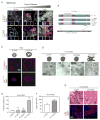
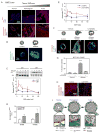
Tumors are characterized by extracellular matrix (ECM) remodeling and stiffening. The importance of ECM remodeling to cancer is appreciated; the relevance of stiffening is less clear. We found that breast tumorigenesis is accompanied by collagen crosslinking, ECM stiffening, and increased focal adhesions. Induction of collagen crosslinking stiffened the ECM, promoted focal adhesions, enhanced PI3 kinase (PI3K) activity, and induced the invasion of an oncogene-initiated epithelium. Inhibition of integrin signaling repressed the invasion of a premalignant epithelium into a stiffened, crosslinked ECM and forced integrin clustering promoted focal adhesions, enhanced PI3K signaling, and induced the invasion of a premalignant epithelium. Consistently, reduction of lysyl oxidase-mediated collagen crosslinking prevented MMTV-Neu-induced fibrosis, decreased focal adhesions and PI3K activity, impeded malignancy, and lowered tumor incidence. These data show how collagen crosslinking can modulate tissue fibrosis and stiffness to force focal adhesions, growth factor signaling and breast malignancy.
Figures
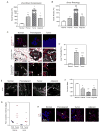

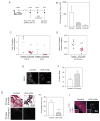
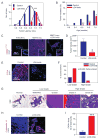
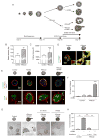
Comment in
-
Environment dictates behaviour.Nat Rev Mol Cell Biol. 2010 Oct;11(10):679. doi: 10.1038/nrm2984. Nat Rev Mol Cell Biol. 2010. PMID: 20861875 No abstract available.
Similar articles
-
Mechanisms by which the extracellular matrix and integrin signaling act to regulate the switch between tumor suppression and tumor promotion.J Mammary Gland Biol Neoplasia. 2011 Sep;16(3):205-19. doi: 10.1007/s10911-011-9226-0. Epub 2011 Aug 7. J Mammary Gland Biol Neoplasia. 2011. PMID: 21822945 Free PMC article. Review.
-
Force engages vinculin and promotes tumor progression by enhancing PI3K activation of phosphatidylinositol (3,4,5)-triphosphate.Cancer Res. 2014 Sep 1;74(17):4597-611. doi: 10.1158/0008-5472.CAN-13-3698. Cancer Res. 2014. PMID: 25183785 Free PMC article.
-
Bi-directional signaling: extracellular matrix and integrin regulation of breast tumor progression.Crit Rev Eukaryot Gene Expr. 2013;23(2):139-57. doi: 10.1615/critreveukargeneexpr.2013006647. Crit Rev Eukaryot Gene Expr. 2013. PMID: 23582036 Free PMC article. Review.
-
Molecular Mechanism Responsible for Fibronectin-controlled Alterations in Matrix Stiffness in Advanced Chronic Liver Fibrogenesis.J Biol Chem. 2016 Jan 1;291(1):72-88. doi: 10.1074/jbc.M115.691519. Epub 2015 Nov 9. J Biol Chem. 2016. PMID: 26553870 Free PMC article.
-
A stiff blow from the stroma: collagen crosslinking drives tumor progression.Cancer Cell. 2009 Dec 8;16(6):455-7. doi: 10.1016/j.ccr.2009.11.013. Cancer Cell. 2009. PMID: 19962663
Cited by
-
Comprehensive characterization of extracellular matrix-related genes in PAAD identified a novel prognostic panel related to clinical outcomes and immune microenvironment: A silico analysis with in vivo and vitro validation.Front Immunol. 2022 Oct 13;13:985911. doi: 10.3389/fimmu.2022.985911. eCollection 2022. Front Immunol. 2022. PMID: 36311789 Free PMC article. Clinical Trial.
-
Contractility, focal adhesion orientation, and stress fiber orientation drive cancer cell polarity and migration along wavy ECM substrates.Proc Natl Acad Sci U S A. 2021 Jun 1;118(22):e2021135118. doi: 10.1073/pnas.2021135118. Proc Natl Acad Sci U S A. 2021. PMID: 34031242 Free PMC article.
-
Physical view on migration modes.Cell Adh Migr. 2015;9(5):367-79. doi: 10.1080/19336918.2015.1066958. Cell Adh Migr. 2015. PMID: 26192136 Free PMC article. Review.
-
The Oncopig as an Emerging Model to Investigate Copper Regulation in Cancer.Int J Mol Sci. 2022 Nov 13;23(22):14012. doi: 10.3390/ijms232214012. Int J Mol Sci. 2022. PMID: 36430490 Free PMC article. Review.
-
Hepatic stellate cells: central modulators of hepatic carcinogenesis.BMC Gastroenterol. 2015 May 27;15:63. doi: 10.1186/s12876-015-0291-5. BMC Gastroenterol. 2015. PMID: 26013123 Free PMC article. Review.
References
-
- Bierie B, Moses HL. Tumour microenvironment: TGFbeta: the molecular Jekyll and Hyde of cancer. Nat Rev Cancer. 2006;6:506–520. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA078731-04/CA/NCI NIH HHS/United States
- R01 CA078731/CA/NCI NIH HHS/United States
- T32HL00795404/HL/NHLBI NIH HHS/United States
- R01 CA078731-07/CA/NCI NIH HHS/United States
- U54CA143836/CA/NCI NIH HHS/United States
- R01 CA078731-08/CA/NCI NIH HHS/United States
- R01 CA078731-06/CA/NCI NIH HHS/United States
- R01 CA138818/CA/NCI NIH HHS/United States
- R01-CA078731/CA/NCI NIH HHS/United States
- R01-CA057621/CA/NCI NIH HHS/United States
- R01 CA057621/CA/NCI NIH HHS/United States
- R01 CA078731-05/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

