Testosterone supplementation reverses sarcopenia in aging through regulation of myostatin, c-Jun NH2-terminal kinase, Notch, and Akt signaling pathways
- PMID: 20022929
- PMCID: PMC2817626
- DOI: 10.1210/en.2009-1177
Testosterone supplementation reverses sarcopenia in aging through regulation of myostatin, c-Jun NH2-terminal kinase, Notch, and Akt signaling pathways
Abstract
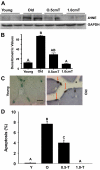
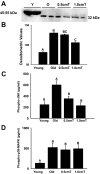
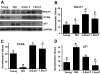
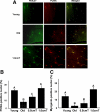
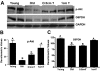
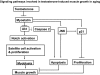
Aging in rodents and humans is characterized by loss of muscle mass (sarcopenia). Testosterone supplementation increases muscle mass in healthy older men. Here, using a mouse model, we investigated the molecular mechanisms by which testosterone prevents sarcopenia and promotes muscle growth in aging. Aged mice of 22 months of age received a single sc injection of GnRH antagonist every 2 wk to suppress endogenous testosterone production and were implanted subdermally under anesthesia with 0.5 or 1.0 cm testosterone-filled implants for 2 months (n = 15/group). Young and old mice (n = 15/group), of 2 and 22 months of age, respectively, received empty implants and were used as controls. Compared with young animals, a significant (P < 0.05) increase in muscle cell apoptosis coupled with a decrease in gastrocnemius muscles weight (by 16.7%) and muscle fiber cross-sectional area, of both fast and slow fiber types, was noted in old mice. Importantly, such age-related changes were fully reversed by higher dose (1 cm) of testosterone treatment. Testosterone treatment effectively suppressed age-specific increases in oxidative stress, processed myostatin levels, activation of c-Jun NH(2)-terminal kinase, and cyclin-dependent kinase inhibitor p21 in aged muscles. Furthermore, it restored age-related decreases in glucose-6-phosphate dehydrogenase levels, phospho-Akt, and Notch signaling. These alterations were associated with satellite cell proliferation and differentiation. Collectively these results suggest involvement of multiple signal transduction pathways in sarcopenia. Testosterone reverses sarcopenia through stimulation of cellular metabolism and survival pathway together with inhibition of death pathway.
Figures
Similar articles
-
Mouse model of testosterone-induced muscle fiber hypertrophy: involvement of p38 mitogen-activated protein kinase-mediated Notch signaling.J Endocrinol. 2009 Apr;201(1):129-39. doi: 10.1677/JOE-08-0476. Epub 2009 Jan 14. J Endocrinol. 2009. PMID: 19144735 Free PMC article.
-
Testosterone is essential for skeletal muscle growth in aged mice in a heterochronic parabiosis model.Cell Tissue Res. 2014 Sep;357(3):815-21. doi: 10.1007/s00441-014-1900-2. Epub 2014 May 24. Cell Tissue Res. 2014. PMID: 24859218 Free PMC article.
-
Involvement of oxidative stress and caspase 2-mediated intrinsic pathway signaling in age-related increase in muscle cell apoptosis in mice.Apoptosis. 2008 Jun;13(6):822-32. doi: 10.1007/s10495-008-0216-7. Apoptosis. 2008. PMID: 18461459 Free PMC article.
-
Molecular mechanisms in aging and current strategies to counteract sarcopenia.Curr Aging Sci. 2010 Jul;3(2):90-101. doi: 10.2174/1874609811003020090. Curr Aging Sci. 2010. PMID: 20158492 Review.
-
PI3 kinase regulation of skeletal muscle hypertrophy and atrophy.Curr Top Microbiol Immunol. 2010;346:267-78. doi: 10.1007/82_2010_78. Curr Top Microbiol Immunol. 2010. PMID: 20593312 Review.
Cited by
-
Sarcopenia and Androgens: A Link between Pathology and Treatment.Front Endocrinol (Lausanne). 2014 Dec 18;5:217. doi: 10.3389/fendo.2014.00217. eCollection 2014. Front Endocrinol (Lausanne). 2014. PMID: 25566189 Free PMC article. Review.
-
Skeletal muscle apoptotic signaling predicts thigh muscle volume and gait speed in community-dwelling older persons: an exploratory study.PLoS One. 2012;7(2):e32829. doi: 10.1371/journal.pone.0032829. Epub 2012 Feb 28. PLoS One. 2012. PMID: 22389725 Free PMC article.
-
Absence of morphological and molecular correlates of sarcopenia in the macaque tongue muscle styloglossus.Exp Gerontol. 2016 Nov;84:40-48. doi: 10.1016/j.exger.2016.08.010. Epub 2016 Aug 24. Exp Gerontol. 2016. PMID: 27566374 Free PMC article.
-
The Roles of Androgens in Humans: Biology, Metabolic Regulation and Health.Int J Mol Sci. 2022 Oct 8;23(19):11952. doi: 10.3390/ijms231911952. Int J Mol Sci. 2022. PMID: 36233256 Free PMC article. Review.
-
Nicotine in combination with a high-fat diet causes intramyocellular mitochondrial abnormalities in male mice.Endocrinology. 2014 Mar;155(3):865-72. doi: 10.1210/en.2013-1795. Epub 2014 Jan 1. Endocrinology. 2014. PMID: 24424058 Free PMC article.
References
-
- Hughes VA, Frontera WR, Roubenoff R, Evans WJ, Singh MA 2002 Longitudinal changes in body composition in older men and women: role of body weight change and physical activity. Am J Clin Nutr 76:473–481 - PubMed
-
- Marzetti E, Leeuwenburgh C 2006 Skeletal muscle apoptosis, sarcopenia and frailty at old age. Exp Gerentol 41:1234–1238 - PubMed
-
- Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, Garry PJ, Lindeman RD 1998 Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 147:755–763 - PubMed
-
- Roubenoff R, Hughes VA 2000 Sarcopenia: current concepts. J. Gerentol A Biol Sci Med Sci 55:M716–M724 - PubMed
-
- Melton 3rd LJ, Khosla S, Crowson CS, O'Connor MK, O'Fallon WM, Riggs BL 2000 Epidemiology of sarcopenia. J Am Geriatr Soc 48:625–630 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

