Th1-Th17 cells mediate protective adaptive immunity against Staphylococcus aureus and Candida albicans infection in mice
- PMID: 20041174
- PMCID: PMC2792038
- DOI: 10.1371/journal.ppat.1000703
Th1-Th17 cells mediate protective adaptive immunity against Staphylococcus aureus and Candida albicans infection in mice
Abstract
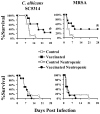
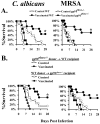
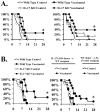
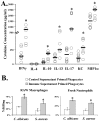
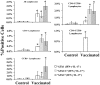
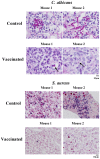
We sought to define protective mechanisms of immunity to Staphylococcus aureus and Candida albicans bloodstream infections in mice immunized with the recombinant N-terminus of Als3p (rAls3p-N) vaccine plus aluminum hydroxide (Al(OH(3)) adjuvant, or adjuvant controls. Deficiency of IFN-gamma but not IL-17A enhanced susceptibility of control mice to both infections. However, vaccine-induced protective immunity against both infections required CD4+ T-cell-derived IFN-gamma and IL-17A, and functional phagocytic effectors. Vaccination primed Th1, Th17, and Th1/17 lymphocytes, which produced pro-inflammatory cytokines that enhanced phagocytic killing of both organisms. Vaccinated, infected mice had increased IFN-gamma, IL-17, and KC, increased neutrophil influx, and decreased organism burden in tissues. In summary, rAls3p-N vaccination induced a Th1/Th17 response, resulting in recruitment and activation of phagocytes at sites of infection, and more effective clearance of S. aureus and C. albicans from tissues. Thus, vaccine-mediated adaptive immunity can protect against both infections by _targeting microbes for destruction by innate effectors.
Conflict of interest statement
BS, ASI, YF, and JEE own equity in NovaDigm Therapeutics, Inc., which is developing vaccine technologies. NovaDigm Therapeutics, Inc. provided no financial support for these studies.
Figures


Similar articles
-
The antifungal vaccine derived from the recombinant N terminus of Als3p protects mice against the bacterium Staphylococcus aureus.Infect Immun. 2008 Oct;76(10):4574-80. doi: 10.1128/IAI.00700-08. Epub 2008 Jul 21. Infect Immun. 2008. PMID: 18644876 Free PMC article.
-
Antibody titer threshold predicts anti-candidal vaccine efficacy even though the mechanism of protection is induction of cell-mediated immunity.J Infect Dis. 2008 Apr 1;197(7):967-71. doi: 10.1086/529204. J Infect Dis. 2008. PMID: 18419471 Free PMC article.
-
Considerable differences in vaccine immunogenicities and efficacies related to the diluent used for aluminum hydroxide adjuvant.Clin Vaccine Immunol. 2008 Mar;15(3):582-4. doi: 10.1128/CVI.00427-07. Epub 2008 Jan 9. Clin Vaccine Immunol. 2008. PMID: 18184821 Free PMC article.
-
Adaptive immune responses to Candida albicans infection.Virulence. 2015;6(4):327-37. doi: 10.1080/21505594.2015.1004977. Epub 2015 Jan 21. Virulence. 2015. PMID: 25607781 Free PMC article. Review.
-
Th17 cells in immunity to Candida albicans.Cell Host Microbe. 2012 May 17;11(5):425-35. doi: 10.1016/j.chom.2012.04.008. Cell Host Microbe. 2012. PMID: 22607796 Free PMC article. Review.
Cited by
-
Cytokine patterns in paediatric patients presenting serious gastrointestinal and respiratory bacterial infections.Cent Eur J Immunol. 2014;39(2):223-7. doi: 10.5114/ceji.2014.43727. Epub 2014 Jun 27. Cent Eur J Immunol. 2014. PMID: 26155128 Free PMC article.
-
Mechanisms by which chronic ethanol feeding impairs the migratory capacity of cutaneous dendritic cells.Alcohol Clin Exp Res. 2013 Dec;37(12):2098-107. doi: 10.1111/acer.12201. Epub 2013 Jul 29. Alcohol Clin Exp Res. 2013. PMID: 23895590 Free PMC article.
-
Identification of Corosolic and Oleanolic Acids as Molecules Antagonizing the Human RORγT Nuclear Receptor Using the Calculated Fingerprints of the Molecular Similarity.Int J Mol Sci. 2022 Feb 8;23(3):1906. doi: 10.3390/ijms23031906. Int J Mol Sci. 2022. PMID: 35163824 Free PMC article.
-
Candida albicans and Staphylococcus Species: A Threatening Twosome.Front Microbiol. 2019 Sep 18;10:2162. doi: 10.3389/fmicb.2019.02162. eCollection 2019. Front Microbiol. 2019. PMID: 31620113 Free PMC article. Review.
-
Development of vaccines for Candida albicans: fighting a skilled transformer.Nat Rev Microbiol. 2013 Dec;11(12):884-91. doi: 10.1038/nrmicro3156. Nat Rev Microbiol. 2013. PMID: 24232568
References
-
- Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, et al. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39:309–317. - PubMed
-
- Chambers HF. Community-associated MRSA–resistance and virulence converge. N Engl J Med. 2005;352:1485–1487. - PubMed
-
- Wilson LS, Reyes CM, Stolpman M, Speckman J, Allen K, et al. The direct cost and incidence of systemic fungal infections. Value Health. 2002;5:26–34. - PubMed
-
- Spellberg B, Filler SG, Edwards JE., Jr Current Treatment Strategies for Disseminated Candidiasis. Clin Infect Dis. 2006;42:244–251. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

