Delivery of factor VIII gene into skeletal muscle cells using lentiviral vector
- PMID: 20046514
- PMCID: PMC2799978
- DOI: 10.3349/ymj.2010.51.1.52
Delivery of factor VIII gene into skeletal muscle cells using lentiviral vector
Abstract
Purpose: This study was designed to investigate whether transduction of lentiviral vectors (LV) carrying human coagulation factor VIII (hFVIII) cDNA into skeletal muscle could increase circulating hFVIII concentrations.
Materials and methods: A LV containing bacterial LacZ gene as a control or human FVIII gene was intramuscularly administered into the thigh muscle of 5 weeks old Sparague-Dawley rats. The plasma human FVIII concentration and neutralizing anti-FVIII antibodies were measured for up to 12 weeks in these experimental animals.
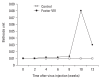
Results: The plasma human FVIII levels in the rats injected with LV carrying FVIII cDNA peaked at post-injection 1st week (5.19 +/- 0.14 ng/mL vs. 0.21 +/- 0.05 ng/mL in control rats , p < 0.05). Elevated hFVIII concentrations were maintained for 4 weeks (2.52 +/- 0.83 ng/mL vs. 0.17 +/- 0.08 ng/mL in control rats, p < 0.05) after a single intramuscular injection. In the Bethesda assay, neutralizing antibodies for FVIII protein were detected only in FVIII-LV injected rats by the 10th week, but not in control rats.
Conclusion: This study suggested that a single administration of an advanced generation LV carrying the human FVIII cDNA resulted in elevation of FVIII level in immune competent rats, and that this gene transfer approach to the skeletal muscle could be an effective tool in treatment of hemophilia A.
Keywords: Gene therapy; hemophilia A; human coagulation factor VIII; lentiviral vector.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures



Similar articles
-
Expression of human coagulation factor VIII in adipocytes transduced with the simian immunodeficiency virus agmTYO1-based vector for hemophilia A gene therapy.Gene Ther. 2004 Feb;11(3):253-9. doi: 10.1038/sj.gt.3302174. Gene Ther. 2004. PMID: 14737084
-
Efficient production of human FVIII in hemophilic mice using lentiviral vectors.Mol Ther. 2003 May;7(5 Pt 1):623-31. doi: 10.1016/s1525-0016(03)00073-x. Mol Ther. 2003. PMID: 12718905
-
Preclinical Development of a Hematopoietic Stem and Progenitor Cell Bioengineered Factor VIII Lentiviral Vector Gene Therapy for Hemophilia A.Hum Gene Ther. 2018 Oct;29(10):1183-1201. doi: 10.1089/hum.2018.137. Hum Gene Ther. 2018. PMID: 30160169 Free PMC article.
-
The Immune Response to the fVIII Gene Therapy in Preclinical Models.Front Immunol. 2020 Apr 15;11:494. doi: 10.3389/fimmu.2020.00494. eCollection 2020. Front Immunol. 2020. PMID: 32351497 Free PMC article. Review.
-
Development of improved factor VIII molecules and new gene transfer approaches for hemophilia A.Curr Gene Ther. 2003 Feb;3(1):27-41. doi: 10.2174/1566523033347417. Curr Gene Ther. 2003. PMID: 12553533 Review.
Cited by
-
Advanced therapies for the treatment of hemophilia: future perspectives.Orphanet J Rare Dis. 2012 Dec 13;7:97. doi: 10.1186/1750-1172-7-97. Orphanet J Rare Dis. 2012. PMID: 23237078 Free PMC article. Review.
-
In Vitro Conditioning of Adipose-Derived Mesenchymal Stem Cells by the Endothelial Microenvironment: Modeling Cell Responsiveness towards Non-Genetic Correction of Haemophilia A.Int J Mol Sci. 2022 Jun 30;23(13):7282. doi: 10.3390/ijms23137282. Int J Mol Sci. 2022. PMID: 35806285 Free PMC article.
-
Cationic nanomicelles for delivery of plasmids encoding interleukin-4 and interleukin-10 for prevention of autoimmune diabetes in mice.Pharm Res. 2012 Mar;29(3):883-97. doi: 10.1007/s11095-011-0616-1. Epub 2011 Nov 11. Pharm Res. 2012. PMID: 22076555
-
Reduced expression of carbonic anhydrase III in skeletal muscles could be linked to muscle fatigue: A rat muscle fatigue model.J Orthop Translat. 2019 Sep 23;22:116-123. doi: 10.1016/j.jot.2019.08.008. eCollection 2020 May. J Orthop Translat. 2019. PMID: 32440507 Free PMC article.
References
-
- Kaufman RJ. Advances toward gene therapy for hemophilia at the millennium. Hum Gene Ther. 1999;10:2091–2107. - PubMed
-
- Levine P. Clinical manifestations an therapy of hemophilia A and B. In: Colman R, Hirsh J, Marder V, Saizman E, editors. Hemostasis and Thrombosis. 2nd ed. Philadelphia, PA: JB Lippincott; 1987. pp. 97–111.
-
- Furie B, Limentani SA, Rosenfield CG. A practical guide to the evaluation and treatment of hemophilia. Blood. 1994;84:3–9. - PubMed
-
- Saenko EL, Ananyeva NM, Shima N, Hauser CA, Pipe SW. The future of recombinant coagulation factors. J Thromb Haemost. 2003;1:922–930. - PubMed
-
- Kreuz W, Ettingshausen CE, Zyschka A, Oldenburg J, Saguer IM, Ehrenforth S, et al. Inhibitor development in previously untreated patients with hemophilia A: a prospective long-term follow-up comparing plasma-derived and recombinant products. Semin Thromb Hemost. 2002;28:285–290. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

