Age and energy intake interact to modify cell stress pathways and stroke outcome
- PMID: 20186857
- PMCID: PMC2844782
- DOI: 10.1002/ana.21798
Age and energy intake interact to modify cell stress pathways and stroke outcome
Abstract
Objective: Age and excessive energy intake/obesity are risk factors for cerebrovascular disease, but it is not known if and how these factors affect the extent of brain damage and outcome in ischemic stroke. We therefore determined the interactions of age and energy intake on the outcome of ischemic brain injury, and elucidated the underlying mechanisms.
Methods: We utilized a novel microchip-based immunoaffinity capillary electrophoresis technology to measure a panel of neurotrophic factors, cytokines, and cellular stress resistance proteins in brain tissue samples from young, middle-aged, and old mice that had been maintained on control or energy-restricted diets prior to middle cerebral artery occlusion and reperfusion.
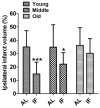
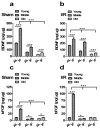
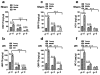
Results: Mortality from focal ischemic stroke was increased with advancing age and reduced by an intermittent fasting (IF) diet. Brain damage and functional impairment were reduced by IF in young and middle-aged mice, but not in old mice. The basal and poststroke levels of neurotrophic factors (brain-derived neurotrophic factor and basic fibroblast growth factor), protein chaperones (heat shock protein 70 and glucose regulated protein 78), and the antioxidant enzyme heme oxygenase-1 were decreased, whereas levels of inflammatory cytokines were increased in the cerebral cortex and striatum of old mice compared with younger mice. IF coordinately increased levels of protective proteins and decreased inflammatory cytokines in young, but not in old mice.
Interpretation: Reduction in dietary energy intake differentially modulates neurotrophic and inflammatory pathways to protect neurons against ischemic injury, and these beneficial effects of IF are compromised during aging, resulting in increased brain damage and poorer functional outcome.
Figures
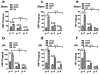
Similar articles
-
Social isolation after stroke leads to depressive-like behavior and decreased BDNF levels in mice.Behav Brain Res. 2014 Mar 1;260:162-70. doi: 10.1016/j.bbr.2013.10.047. Epub 2013 Nov 5. Behav Brain Res. 2014. PMID: 24211537 Free PMC article.
-
Resveratrol attenuates ischemic brain damage in the delayed phase after stroke and induces messenger RNA and protein express for angiogenic factors.J Vasc Surg. 2008 Sep;48(3):709-14. doi: 10.1016/j.jvs.2008.04.007. Epub 2008 Jun 24. J Vasc Surg. 2008. PMID: 18572362
-
Exercise preconditioning ameliorates inflammatory injury in ischemic rats during reperfusion.Acta Neuropathol. 2005 Mar;109(3):237-46. doi: 10.1007/s00401-004-0943-y. Epub 2004 Dec 23. Acta Neuropathol. 2005. PMID: 15616790
-
Opposing effects of glucose on stroke and reperfusion injury: acidosis, oxidative stress, and energy metabolism.Stroke. 2014 Jun;45(6):1881-6. doi: 10.1161/STROKEAHA.114.004889. Epub 2014 Apr 17. Stroke. 2014. PMID: 24743441 Free PMC article. Review. No abstract available.
-
Age-related changes in brain support cells: Implications for stroke severity.Neurochem Int. 2013 Oct;63(4):291-301. doi: 10.1016/j.neuint.2013.06.013. Epub 2013 Jun 28. Neurochem Int. 2013. PMID: 23811611 Free PMC article. Review.
Cited by
-
Salvianolate lyophilized injection promotes post-stroke functional recovery via the activation of VEGF and BDNF-TrkB-CREB signaling pathway.Int J Clin Exp Med. 2015 Jan 15;8(1):108-22. eCollection 2015. Int J Clin Exp Med. 2015. PMID: 25784980 Free PMC article.
-
Stroke outcome in the ketogenic state--a systematic review of the animal data.J Neurochem. 2012 Nov;123 Suppl 2(0 2):52-7. doi: 10.1111/j.1471-4159.2012.07943.x. J Neurochem. 2012. PMID: 23050642 Free PMC article. Review.
-
Fad Diets: Hype or Hope?Curr Nutr Rep. 2018 Dec;7(4):310-323. doi: 10.1007/s13668-018-0242-1. Curr Nutr Rep. 2018. PMID: 30168044 Review.
-
ER calcium and Alzheimer's disease: in a state of flux.Sci Signal. 2010 Mar 23;3(114):pe10. doi: 10.1126/scisignal.3114pe10. Sci Signal. 2010. PMID: 20332425 Free PMC article. Review.
-
Perspective: Does brown fat protect against diseases of aging?Ageing Res Rev. 2010 Jan;9(1):69-76. doi: 10.1016/j.arr.2009.11.004. Epub 2009 Dec 5. Ageing Res Rev. 2010. PMID: 19969105 Free PMC article. Review.
References
-
- Shuaib A, Boyle C. Stroke in the elderly. Curr Opin Neurol. 1994;7:41–47. - PubMed
-
- Zheng Z, Lee JE, Yenari M. Stroke: molecular mechanisms and potential _targets for treatment. Curr Mol Med. 2003;3:361–372. - PubMed
-
- Endres M, Fan G, Hirt L, et al. Ischemic brain damage in mice after selectively modifying BDNF or NT4 gene expression. J Cereb Blood Flow Metab. 2000;20:139–144. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

