Phase I/II study of combination therapy with sorafenib, idarubicin, and cytarabine in younger patients with acute myeloid leukemia
- PMID: 20212254
- PMCID: PMC2930809
- DOI: 10.1200/JCO.2009.25.4888
Phase I/II study of combination therapy with sorafenib, idarubicin, and cytarabine in younger patients with acute myeloid leukemia
Abstract
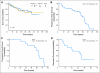
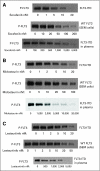
PURPOSE To determine the efficacy and toxicity of the combination of sorafenib, cytarabine, and idarubicin in patients with acute myeloid leukemia (AML) younger than age 65 years. PATIENTS AND METHODS In the phase I part of the study, 10 patients with relapsed AML were treated with escalating doses of sorafenib with chemotherapy to establish the feasibility of the combination. We then treated 51 patients (median age, 53 years; range, 18 to 65 years) who had previously untreated AML with cytarabine at 1.5 g/m(2) by continuous intravenous (IV) infusion daily for 4 days (3 days if > 60 years of age), idarubicin at 12 mg/m(2) IV daily for 3 days, and sorafenib at 400 mg orally twice daily for 7 days. RESULTS Overall, 38 (75%) patients have achieved a complete remission (CR), including 14 (93%) of 15 patients with mutated FMS-like tyrosine kinase-3 (FLT3; the 15th patient had complete remission with incomplete platelet recovery [CRp]) and 24 (66%) of 36 patients with FLT3 wild-type (WT) disease (three additional FLT3-WT patients had CRp). FLT3-mutated patients were more likely to achieve a CR than FLT3-WT patients (P = .033). With a median follow-up of 54 weeks (range, 8 to 87 weeks), the probability of survival at 1 year is 74%. Among the FLT3-mutated patients, 10 have relapsed and five remain in CR with a median follow-up of 62 weeks (range, 10 to 76 weeks). Plasma inhibitory assay demonstrated an on-_target effect on FLT3 kinase activity. CONCLUSION Sorafenib can be safely combined with chemotherapy, produces a high CR rate in FLT3-mutated patients, and inhibits FLT3 signaling.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures

Similar articles
-
Patterns of molecular response to and relapse after combination of sorafenib, idarubicin, and cytarabine in patients with FLT3 mutant acute myeloid leukemia.Clin Lymphoma Myeloma Leuk. 2011 Aug;11(4):361-6. doi: 10.1016/j.clml.2011.06.007. Clin Lymphoma Myeloma Leuk. 2011. PMID: 21816375 Free PMC article.
-
Phase I pharmacokinetic and pharmacodynamic study of the multikinase inhibitor sorafenib in combination with clofarabine and cytarabine in pediatric relapsed/refractory leukemia.J Clin Oncol. 2011 Aug 20;29(24):3293-300. doi: 10.1200/JCO.2011.34.7427. Epub 2011 Jul 18. J Clin Oncol. 2011. PMID: 21768474 Free PMC article. Clinical Trial.
-
Phase 2 study of azacytidine plus sorafenib in patients with acute myeloid leukemia and FLT-3 internal tandem duplication mutation.Blood. 2013 Jun 6;121(23):4655-62. doi: 10.1182/blood-2013-01-480228. Epub 2013 Apr 23. Blood. 2013. PMID: 23613521 Free PMC article. Clinical Trial.
-
[Sorafenib in combination with chemotherapy in the induction therapy for FLT3-ITD positive acute monocytic leukemia: a case report and literature review].Zhonghua Xue Ye Xue Za Zhi. 2011 Jan;32(1):8-11. Zhonghua Xue Ye Xue Za Zhi. 2011. PMID: 21429393 Review. Chinese.
-
Midostaurin in Combination With Standard Chemotherapy for Treatment of Newly Diagnosed FMS-Like Tyrosine Kinase 3 (FLT3) Mutation-Positive Acute Myeloid Leukemia.Ann Pharmacother. 2018 Apr;52(4):364-369. doi: 10.1177/1060028017747900. Epub 2017 Dec 12. Ann Pharmacother. 2018. PMID: 29231051 Review.
Cited by
-
FLT3 mutated acute myeloid leukemia: 2021 treatment algorithm.Blood Cancer J. 2021 May 27;11(5):104. doi: 10.1038/s41408-021-00495-3. Blood Cancer J. 2021. PMID: 34045454 Free PMC article. Review.
-
Phase IB study of the FLT3 kinase inhibitor midostaurin with chemotherapy in younger newly diagnosed adult patients with acute myeloid leukemia.Leukemia. 2012 Sep;26(9):2061-8. doi: 10.1038/leu.2012.115. Epub 2012 Apr 27. Leukemia. 2012. PMID: 22627678 Free PMC article. Clinical Trial.
-
'Acute myeloid leukemia: a comprehensive review and 2016 update'.Blood Cancer J. 2016 Jul 1;6(7):e441. doi: 10.1038/bcj.2016.50. Blood Cancer J. 2016. PMID: 27367478 Free PMC article. Review.
-
p53 activation of mesenchymal stromal cells partially abrogates microenvironment-mediated resistance to FLT3 inhibition in AML through HIF-1α-mediated down-regulation of CXCL12.Blood. 2011 Oct 20;118(16):4431-9. doi: 10.1182/blood-2011-02-334136. Epub 2011 Aug 25. Blood. 2011. PMID: 21868571 Free PMC article.
-
New and Emerging _targeted Therapies for Pediatric Acute Myeloid Leukemia (AML).Children (Basel). 2020 Feb 10;7(2):12. doi: 10.3390/children7020012. Children (Basel). 2020. PMID: 32050659 Free PMC article. Review.
References
-
- Gilliland DG, Griffin JD. The roles of FLT3 in hematopoiesis and leukemia. Blood. 2002;100:1532–1542. - PubMed
-
- Small D. FLT3 mutations: Biology and treatment. Hematology Am Soc Hematol Educ Program. 2006:178–184. - PubMed
-
- Kottaridis PD, Gale RE, Frew ME, et al. The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: Analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials. Blood. 2001;98:1752–1759. - PubMed
-
- Fröhling S, Schlenk RF, Breitruck J, et al. Prognostic significance of activating FLT3 mutations in younger adults (16 to 60 years) with acute myeloid leukemia and normal cytogenetics: A study of the AML Study Group Ulm. Blood. 2002;100:4372–4380. - PubMed
-
- Thiede C, Steudel C, Mohr B, et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: Association with FAB subtypes and identification of subgroups with poor prognosis. Blood. 2002;99:4326–4335. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

