Randomized clinical trial of quick-release bromocriptine among patients with type 2 diabetes on overall safety and cardiovascular outcomes
- PMID: 20332352
- PMCID: PMC2890350
- DOI: 10.2337/dc09-2009
Randomized clinical trial of quick-release bromocriptine among patients with type 2 diabetes on overall safety and cardiovascular outcomes
Erratum in
-
Erratum. Randomized Clinical Trial of Quick-Release Bromocriptine Among Patients With Type 2 Diabetes on Overall Safety and Cardiovascular Outcomes.Diabetes Care. 2016 Oct;39(10):1846. doi: 10.2337/dc16-er10. Diabetes Care. 2016. PMID: 27660123 Free PMC article. No abstract available.
Abstract
Objective: Quick-release bromocriptine (bromocriptine-QR), a D2 dopamine receptor agonist, is indicated as a treatment for type 2 diabetes. The Cycloset Safety Trial, a 52-week, randomized, double-blind, multicenter trial, evaluated the overall safety and cardiovascular safety of this novel therapy for type 2 diabetes.
Research design and methods: A total of 3,095 patients with type 2 diabetes were randomized 2:1 to bromocriptine-QR or placebo in conjunction with the patient's usual diabetes therapy (diet controlled only or up to two antidiabetes medications, including insulin). The all-cause-safety end point was the occurrence of any serious adverse event (SAE), with a hazard ratio (HR) noninferiority margin of 1.5. In a prespecified analysis, the frequency of cardiovascular disease (CVD) events defined as a composite of myocardial infarction, stroke, coronary revascularization, and hospitalization for angina or congestive heart failure was evaluated using modified intent-to-treat analysis (clinicaltrials.gov, NCT00377676).
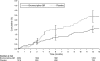
Results: In the bromocriptine-QR group, 176 (8.6%) people reported SAEs compared with 98 (9.6%) in the placebo group (HR 1.02 [96% one-sided CI 1.27]). Fewer people reported a CVD end point in the bromocriptine-QR group versus the placebo group (37 [1.8%] vs. 32 [3.2%], respecively) (HR 0.60 [95% two-sided CI 0.35-0.96]). Nausea was the most commonly reported adverse event in the bromocriptine-QR group.
Conclusions: The frequency of SAEs was comparable between the treatment arms. Compared with patients in the placebo arm, fewer patients taking bromocriptine-QR experienced a cardiovascular end point.
Figures
Similar articles
-
Effect of bromocriptine-QR (a quick-release formulation of bromocriptine mesylate) on major adverse cardiovascular events in type 2 diabetes subjects.J Am Heart Assoc. 2012 Oct;1(5):e002279. doi: 10.1161/JAHA.112.002279. Epub 2012 Oct 25. J Am Heart Assoc. 2012. PMID: 23316290 Free PMC article. Clinical Trial.
-
Impact of bromocriptine-QR therapy on cardiovascular outcomes in type 2 diabetes mellitus subjects on metformin.Postgrad Med. 2016 Nov;128(8):761-769. doi: 10.1080/00325481.2016.1243003. Epub 2016 Oct 11. Postgrad Med. 2016. PMID: 27687032 Clinical Trial.
-
Timed Bromocriptine-QR Therapy Reduces Progression of Cardiovascular Disease and Dysglycemia in Subjects with Well-Controlled Type 2 Diabetes Mellitus.J Diabetes Res. 2015;2015:157698. doi: 10.1155/2015/157698. Epub 2015 Apr 28. J Diabetes Res. 2015. PMID: 26060823 Free PMC article. Clinical Trial.
-
Efficacy and Safety of Bromocriptine-QR as an Adjunctive Therapy on Glycemic Control in Subjects with Uncontrolled Type 2 Diabetes Mellitus: A Systematic Review and Meta-analysis.J ASEAN Fed Endocr Soc. 2024;39(1):95-105. doi: 10.15605/jafes.039.01.19. Epub 2024 Feb 21. J ASEAN Fed Endocr Soc. 2024. PMID: 38863918 Free PMC article. Review.
-
Late phase completed clinical trials investigating bromocriptine mesylate quick release as treatment of type 2 diabetes mellitus.Expert Opin Pharmacother. 2021 Feb;22(2):241-247. doi: 10.1080/14656566.2020.1825683. Epub 2020 Oct 8. Expert Opin Pharmacother. 2021. PMID: 33030357 Review.
Cited by
-
Mechanisms of current therapies for diabetes mellitus type 2.Adv Physiol Educ. 2012 Dec;36(4):275-83. doi: 10.1152/advan.00094.2012. Adv Physiol Educ. 2012. PMID: 23209008 Free PMC article. Review.
-
Dopamine 2 agonists for the management of type 2 diabetes: a systematic review and meta-analysis.J Diabetes Metab Disord. 2023 May 12;22(2):931-943. doi: 10.1007/s40200-023-01230-4. eCollection 2023 Dec. J Diabetes Metab Disord. 2023. PMID: 37975084 Free PMC article. Review.
-
Drug treatment of type 2 diabetes mellitus in patients for whom metformin is contraindicated.Diabetes Metab Syndr Obes. 2014 Jan 18;7:15-24. doi: 10.2147/DMSO.S38753. eCollection 2014 Jan 18. Diabetes Metab Syndr Obes. 2014. PMID: 24465132 Free PMC article. Review.
-
The emergence of cardiodiabetology.Cardiovasc Endocrinol. 2017 Feb 15;6(1):3-7. doi: 10.1097/XCE.0000000000000117. eCollection 2017 Mar. Cardiovasc Endocrinol. 2017. PMID: 31646112 Free PMC article. Review. No abstract available.
-
Type 2 diabetes mellitus--current therapies and the emergence of surgical options.Nat Rev Endocrinol. 2011 Feb 8;7(7):408-19. doi: 10.1038/nrendo.2011.10. Nat Rev Endocrinol. 2011. PMID: 21301486 Review.
References
-
- Roglic G, Unwin N, Bennett PH, Mathers C, Tuomilehto J, Nag S, Connolly V, King H: The burden of mortality attributable to diabetes: realistic estimates for the year 2000. Diabetes Care 2005; 28: 2130–2135 - PubMed
-
- Diabetes Facts and Figures. World Health Ogranization. Available from http://www.who.int/diabetes/facts/en/. Accessed
-
- Nissen SE, Wolski K: Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 2007; 356: 2457–2471 - PubMed
-
- Olsson J, Lindberg G, Gottsäter M, Lindwall K, Sjöstrand A, Tisell A, Melander A: Increased mortality in Type II diabetic patients using sulphonylurea and metformin in combination: a population-based observational study. Diabetologia 2000; 43: 558–560 - PubMed
-
- Walker AM, McAfee AT, Koro C: Studies of diabetes, thiazolidinediones, and coronary heart disease. Pharmacoepidemiol Drug Saf 2007; 16: 1313–1314 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

