Real-time PCR expression profiling of genes encoding potential virulence factors in Candida albicans biofilms: identification of model-dependent and -independent gene expression
- PMID: 20398368
- PMCID: PMC2862034
- DOI: 10.1186/1471-2180-10-114
Real-time PCR expression profiling of genes encoding potential virulence factors in Candida albicans biofilms: identification of model-dependent and -independent gene expression
Abstract
Background: Candida albicans infections are often associated with biofilm formation. Previous work demonstrated that the expression of HWP1 (hyphal wall protein) and of genes belonging to the ALS (agglutinin-like sequence), SAP (secreted aspartyl protease), PLB (phospholipase B) and LIP (lipase) gene families is associated with biofilm growth on mucosal surfaces. We investigated using real-time PCR whether genes encoding potential virulence factors are also highly expressed in biofilms associated with abiotic surfaces. For this, C. albicans biofilms were grown on silicone in microtiter plates (MTP) or in the Centres for Disease Control (CDC) reactor, on polyurethane in an in vivo subcutaneous catheter rat (SCR) model, and on mucosal surfaces in the reconstituted human epithelium (RHE) model.
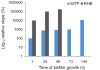
Results: HWP1 and genes belonging to the ALS, SAP, PLB and LIP gene families were constitutively expressed in C. albicans biofilms. ALS1-5 were upregulated in all model systems, while ALS9 was mostly downregulated. ALS6 and HWP1 were overexpressed in all models except in the RHE and MTP, respectively. The expression levels of SAP1 were more pronounced in both in vitro models, while those of SAP2, SAP4 and SAP6 were higher in the in vivo model. Furthermore, SAP5 was highly upregulated in the in vivo and RHE models. For SAP9 and SAP10 similar gene expression levels were observed in all model systems. PLB genes were not considerably upregulated in biofilms, while LIP1-3, LIP5-7 and LIP9-10 were highly overexpressed in both in vitro models. Furthermore, an elevated lipase activity was detected in supernatans of biofilms grown in the MTP and RHE model.
Conclusions: Our findings show that HWP1 and most of the genes belonging to the ALS, SAP and LIP gene families are upregulated in C. albicans biofilms. Comparison of the fold expression between the various model systems revealed similar expression levels for some genes, while for others model-dependent expression levels were observed. This suggests that data obtained in one biofilm model cannot be extrapolated to other model systems. Therefore, the need to use multiple model systems when studying the expression of genes encoding potential virulence factors in C. albicans biofilms is highlighted.
Figures
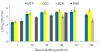

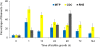
Similar articles
-
Expression of SAP5 and SAP9 in Candida albicans biofilms: comparison of bloodstream isolates with isolates from other sources.Med Mycol. 2013 Nov;51(8):892-6. doi: 10.3109/13693786.2013.824623. Epub 2013 Aug 23. Med Mycol. 2013. PMID: 23971863
-
RT-PCR detection of Candida albicans ALS gene expression in the reconstituted human epithelium (RHE) model of oral candidiasis and in model biofilms.Microbiology (Reading). 2004 Feb;150(Pt 2):267-275. doi: 10.1099/mic.0.26699-0. Microbiology (Reading). 2004. PMID: 14766904
-
Quantitative expression of the Candida albicans secreted aspartyl proteinase gene family in human oral and vaginal candidiasis.Microbiology (Reading). 2008 Nov;154(Pt 11):3266-3280. doi: 10.1099/mic.0.2008/022293-0. Microbiology (Reading). 2008. PMID: 18957581 Free PMC article.
-
Host-pathogen interactions and virulence-associated genes during Candida albicans oral infections.Int J Med Microbiol. 2011 Jun;301(5):417-22. doi: 10.1016/j.ijmm.2011.04.009. Epub 2011 May 8. Int J Med Microbiol. 2011. PMID: 21555244 Review.
-
Candida albicans biofilms: development, regulation, and molecular mechanisms.Microbes Infect. 2016 May;18(5):310-21. doi: 10.1016/j.micinf.2016.01.002. Epub 2016 Jan 22. Microbes Infect. 2016. PMID: 26806384 Free PMC article. Review.
Cited by
-
Nanoparticles as Potential Novel Therapies for Urinary Tract Infections.Front Cell Infect Microbiol. 2021 Apr 19;11:656496. doi: 10.3389/fcimb.2021.656496. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 33954121 Free PMC article. Review.
-
In vitro evaluation of antifungal activity of monolaurin against Candida albicans biofilms.PeerJ. 2016 Jun 22;4:e2148. doi: 10.7717/peerj.2148. eCollection 2016. PeerJ. 2016. PMID: 27366648 Free PMC article.
-
Synergistic Antibiofilm Effects of Pseudolaric Acid A Combined with Fluconazole against Candida albicans via Inhibition of Adhesion and Yeast-To-Hypha Transition.Microbiol Spectr. 2022 Apr 27;10(2):e0147821. doi: 10.1128/spectrum.01478-21. Epub 2022 Mar 17. Microbiol Spectr. 2022. PMID: 35297651 Free PMC article.
-
In vitro infection models to study fungal-host interactions.FEMS Microbiol Rev. 2021 Sep 8;45(5):fuab005. doi: 10.1093/femsre/fuab005. FEMS Microbiol Rev. 2021. PMID: 33524102 Free PMC article. Review.
-
Carbon source-induced reprogramming of the cell wall proteome and secretome modulates the adherence and drug resistance of the fungal pathogen Candida albicans.Proteomics. 2012 Nov;12(21):3164-79. doi: 10.1002/pmic.201200228. Proteomics. 2012. PMID: 22997008 Free PMC article.
References
-
- Odds FC. Meeting Candida and Candidiosis. 2. Bailliere Tindall London UK; 1988.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

