Nurse led, home based self help treatment for patients in primary care with chronic fatigue syndrome: randomised controlled trial
- PMID: 20418251
- PMCID: PMC2859122
- DOI: 10.1136/bmj.c1777
Nurse led, home based self help treatment for patients in primary care with chronic fatigue syndrome: randomised controlled trial
Abstract
Objective: To evaluate the effectiveness of home delivered pragmatic rehabilitation-a programme of gradually increasing activity designed collaboratively by the patient and the therapist-and supportive listening-an approach based on non-directive counselling-for patients in primary care with chronic fatigue syndrome/myalgic encephalomyelitis or encephalitis (CFS/ME).
Design: Single blind, randomised, controlled trial.
Setting: 186 general practices across the north west of England between February 2005 and May 2007.
Participants: 296 patients aged 18 or over with CFS/ME (median illness duration seven years) diagnosed using the Oxford criteria.
Interventions: Participants were randomly allocated to pragmatic rehabilitation, supportive listening, or general practitioner treatment as usual. Both therapies were delivered at home in 10 sessions over 18 weeks by one of three adult specialty general nurses who had received four months' training, including supervised practice, in each of the interventions. GP treatment as usual was unconstrained except that patients were not to be referred for systematic psychological therapies during the treatment period. Main outcome measures The primary clinical outcomes were fatigue and physical functioning at the end of treatment (20 weeks) and 70 weeks from recruitment compared with GP treatment as usual. Lower fatigue scores and higher physical functioning scores denote better outcomes.
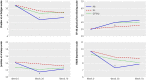
Results: A total of 257 (87%) of the 296 patients who entered the trial were assessed at 70 weeks, the primary outcome point. Analysis was on an intention to treat basis, with robust treatment effects estimated after adjustment for missing data using probability weights. Immediately after treatment (at 20 weeks), patients allocated to pragmatic rehabilitation (n=95) had significantly improved fatigue (effect estimate -1.18, 95% confidence interval -2.18 to -0.18; P=0.021) but not physical functioning (-0.18, 95% CI -5.88 to +5.52; P=0.950) compared with patients allocated to treatment as usual (n=100). At one year after finishing treatment (70 weeks), there were no statistically significant differences in fatigue or physical functioning between patients allocated to pragmatic rehabilitation and those on treatment as usual (-1.00, 95% CI -2.10 to +0.11; P=0.076 and +2.57, 95% CI 3.90 to +9.03; P=0.435). At 20 weeks, patients allocated to supportive listening (n=101) had poorer physical functioning than those allocated to treatment as usual (-7.54, 95% CI -12.76 to -2.33; P=0.005) and no difference in fatigue. At 70 weeks, patients allocated to supportive listening did not differ significantly from those allocated to treatment as usual on either primary outcome.
Conclusions: For patients with CFS/ME in primary care, pragmatic rehabilitation delivered by trained nurse therapists improves fatigue in the short term compared with unconstrained GP treatment as usual, but the effect is small and not statistically significant at one year follow-up. Supportive listening delivered by trained nurse therapists is not an effective treatment for CFS/ME. Trial registration International Standard Randomised Controlled Trial Number IRCTN74156610.
Conflict of interest statement
Competing interests: All authors have completed the Unified Competing Interest form at
Figures
Comment in
-
Pragmatic rehabilitation for chronic fatigue syndrome.BMJ. 2010 Apr 23;340:c1799. doi: 10.1136/bmj.c1799. BMJ. 2010. PMID: 20418252 No abstract available.
-
Nurse-delivered, home-based pragmatic rehabilitation has a short-term effect on improving fatigue in people with chronic fatigue syndrome compared with usual GP care, but effects were not sustained at 1 year.Evid Based Nurs. 2010 Oct;13(4):125-6. doi: 10.1136/ebn1086. Evid Based Nurs. 2010. PMID: 20855345 No abstract available.
Similar articles
-
Fatigue Intervention by Nurses Evaluation--the FINE Trial. A randomised controlled trial of nurse led self-help treatment for patients in primary care with chronic fatigue syndrome: study protocol. [ISRCTN74156610].BMC Med. 2006 Apr 7;4:9. doi: 10.1186/1741-7015-4-9. BMC Med. 2006. PMID: 16603058 Free PMC article.
-
Cost-effectiveness of supported self-management for CFS/ME patients in primary care.BMC Fam Pract. 2013 Jan 18;14:12. doi: 10.1186/1471-2296-14-12. BMC Fam Pract. 2013. PMID: 23327355 Free PMC article. Clinical Trial.
-
Group cognitive-behavioural programme to reduce the impact of rheumatoid arthritis fatigue: the RAFT RCT with economic and qualitative evaluations.Health Technol Assess. 2019 Oct;23(57):1-130. doi: 10.3310/hta23570. Health Technol Assess. 2019. PMID: 31601357 Free PMC article. Clinical Trial.
-
Exercise therapy for chronic fatigue syndrome.Cochrane Database Syst Rev. 2017 Apr 25;4(4):CD003200. doi: 10.1002/14651858.CD003200.pub7. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2019 Oct 02;10:CD003200. doi: 10.1002/14651858.CD003200.pub8 PMID: 28444695 Free PMC article. Updated. Review.
-
Exercise therapy for chronic fatigue syndrome.Cochrane Database Syst Rev. 2016 Dec 20;12(12):CD003200. doi: 10.1002/14651858.CD003200.pub6. Cochrane Database Syst Rev. 2016. Update in: Cochrane Database Syst Rev. 2017 Apr 25;4:CD003200. doi: 10.1002/14651858.CD003200.pub7 PMID: 27995604 Free PMC article. Updated. Review.
Cited by
-
Treating medically unexplained symptoms via improving access to psychological therapy (IAPT): major limitations identified.BMC Psychol. 2020 Feb 5;8(1):13. doi: 10.1186/s40359-020-0380-2. BMC Psychol. 2020. PMID: 32020880 Free PMC article. Review.
-
A comparison of approaches for combining predictive markers for personalised treatment recommendations.Trials. 2021 Jan 6;22(1):20. doi: 10.1186/s13063-020-04901-2. Trials. 2021. PMID: 33407760 Free PMC article. Clinical Trial.
-
Chronic fatigue syndrome and quality of life.Patient Relat Outcome Meas. 2018 Aug 1;9:253-262. doi: 10.2147/PROM.S155642. eCollection 2018. Patient Relat Outcome Meas. 2018. PMID: 30123017 Free PMC article. Review.
-
The Draft Report by the Institute for Quality and Efficiency in Healthcare Does Not Provide Any Evidence That Graded Exercise Therapy and Cognitive Behavioral Therapy Are Safe and Effective Treatments for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome.Diseases. 2023 Jan 16;11(1):11. doi: 10.3390/diseases11010011. Diseases. 2023. PMID: 36648876 Free PMC article. Review.
-
Differential Metabolites and Metabolic Pathways Involved in Aerobic Exercise Improvement of Chronic Fatigue Symptoms in Adolescents Based on Gas Chromatography-Mass Spectrometry.Int J Environ Res Public Health. 2022 Feb 18;19(4):2377. doi: 10.3390/ijerph19042377. Int J Environ Res Public Health. 2022. PMID: 35206569 Free PMC article. Clinical Trial.
References
-
- Fukuda K, Straus SE, Hickie I, Sharpe MC, Dobbins JG, Komaroff A, et al. The chronic fatigue syndrome—a comprehensive approach to its definition and study. Ann Intern Med 1994;121:953-9. - PubMed
-
- Komaroff AL, Fagioli LR, Doolittle TH, Gandek B, Gleit MA, Guerriero RT, et al. Health status in patients with chronic fatigue syndrome and in general population and disease comparison groups. Am J Med 1996;101:281-90. - PubMed
-
- Bombardier CH, Buchwald D. Chronic fatigue, chronic fatigue syndrome, and fibromyalgia—disability and health-care use. Med Care 1996;34:924-30. - PubMed
-
- McCrone P, Darbishire L, Ridsdale L, Seed P. The economic cost of chronic fatigue and chronic fatigue syndrome in UK primary care. Psychol Med 2003;33:253-61. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
