Radiation therapy induces circulating serum Hsp72 in patients with prostate cancer
- PMID: 20430459
- PMCID: PMC2883632
- DOI: 10.1016/j.radonc.2010.03.024
Radiation therapy induces circulating serum Hsp72 in patients with prostate cancer
Abstract
Background and purpose: Hsp72 found in the extracellular milieu has been shown to play an important role in immune regulation. The impact of common cancer therapies on extracellular release of Hsp72 however, has been to date undefined.
Materials and methods: Serum from 13 patients undergoing radiation therapy (XRT) for prostate cancer with or without hormonal therapy (ADT) was measured for levels of circulating serum Hsp72 and pro-inflammatory cytokines (IL-6 and TNF-alpha) using the classical sandwich ELISA technique and the relative expression of CD8(+) T lymphocytes and natural killer (NK) cells was measured using flow cytometry. Mouse orthotopic xenograft of human prostate cancer tumors (DU-145 and PC-3) were used to validate and further characterize the response noted in the clinical setting. The biological significance of tumor released Hsp72 was studied in human dendritic cells (DC) in vitro.
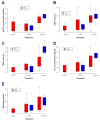
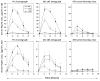
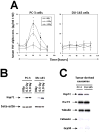
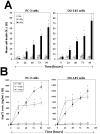
Results: Circulating serum Hsp72 levels increased an average of 3.5-fold (median per patient 4.8-fold) with XRT but not with ADT (p=0.0002). Increases in IL-6 (3.3-fold), TNF-alpha (1.8-fold), CD8(+) CTL (2.1-fold) and NK cells (3.2-fold) also occurred. Using PC-3 and DU-145 human prostate cancer xenograft models in mice, we confirmed that XRT induces Hsp72 release primarily from implanted tumors. In vitro studies using supernatant recovered from irradiated human prostate cancer cells point to exosomes containing Hsp72 as a possible stimulator of pro-inflammatory cytokine production and costimulatory molecules expression in human DC.
Conclusions: The current study confirms for the first time in an actual clinical setting elevation of circulating serum Hsp72 with XRT. The accompanying studies in mice and in vitro identify the released exosomes containing Hsp72 as playing a pivotal role in stimulating pro-inflammatory immune responses. These findings, if validated, may lead to new treatment paradigms for common human malignancies.
Copyright 2010 Elsevier Ireland Ltd. All rights reserved.
Conflict of interest statement
The authors have no conflict of interest.
Figures
Similar articles
-
Tumor necrosis factor gene-engineered J558 tumor cell-released exosomes stimulate tumor antigen P1A-specific CD8+ CTL responses and antitumor immunity.Cancer Biother Radiopharm. 2010 Feb;25(1):21-8. doi: 10.1089/cbr.2009.0714. Cancer Biother Radiopharm. 2010. PMID: 20187793
-
Membrane-bound HSP70-engineered myeloma cell-derived exosomes stimulate more efficient CD8(+) CTL- and NK-mediated antitumour immunity than exosomes released from heat-shocked tumour cells expressing cytoplasmic HSP70.J Cell Mol Med. 2010 Nov;14(11):2655-66. doi: 10.1111/j.1582-4934.2009.00851.x. J Cell Mol Med. 2010. PMID: 19627400 Free PMC article.
-
Effects of temperature-humidity index and chromium supplementation on antioxidant capacity, heat shock protein 72, and cytokine responses of lactating cows.J Anim Sci. 2014 Jul;92(7):3026-34. doi: 10.2527/jas.2013-6932. Epub 2014 May 30. J Anim Sci. 2014. PMID: 24879765
-
Hsp72 release: mechanisms and methodologies.Methods. 2007 Nov;43(3):194-8. doi: 10.1016/j.ymeth.2007.06.002. Methods. 2007. Retraction in: Methods. 2009 Mar;47(3):223. doi: 10.1016/j.ymeth.2008.11.003 PMID: 17920515 Retracted. Review.
-
Mechanisms of stress-induced cellular HSP72 release: implications for exercise-induced increases in extracellular HSP72.Exerc Immunol Rev. 2005;11:46-52. Exerc Immunol Rev. 2005. PMID: 16385843 Review.
Cited by
-
A Clinician's Guide to Cancer-Derived Exosomes: Immune Interactions and Therapeutic Implications.Front Immunol. 2020 Jul 22;11:1612. doi: 10.3389/fimmu.2020.01612. eCollection 2020. Front Immunol. 2020. PMID: 32793238 Free PMC article. Review.
-
Changes in biomarkers of inflammation and angiogenesis during androgen deprivation therapy for prostate cancer.Oncologist. 2012;17(2):212-9. doi: 10.1634/theoncologist.2011-0321. Epub 2012 Feb 2. Oncologist. 2012. PMID: 22302227 Free PMC article.
-
Impact of curative radiotherapy on the immune status of patients with localized prostate cancer.Oncoimmunology. 2018 Aug 27;7(11):e1496881. doi: 10.1080/2162402X.2018.1496881. eCollection 2018. Oncoimmunology. 2018. PMID: 30393582 Free PMC article.
-
Exosomes in Prostate Cancer Diagnosis, Prognosis and Therapy.Int J Mol Sci. 2020 Mar 19;21(6):2118. doi: 10.3390/ijms21062118. Int J Mol Sci. 2020. PMID: 32204455 Free PMC article. Review.
-
A single nucleotide polymorphism in inflammatory gene RNASEL predicts outcome after radiation therapy for localized prostate cancer.Clin Cancer Res. 2013 Mar 15;19(6):1612-9. doi: 10.1158/1078-0432.CCR-12-2718. Epub 2013 Feb 4. Clin Cancer Res. 2013. PMID: 23382116 Free PMC article.
References
-
- Beckmann RP, Mizzen LE, Welch WJ. Interaction of Hsp 70 with newly synthesized proteins: implications for protein folding and assembly. Science. 1990;248:850–854. - PubMed
-
- Gething MJ, Sambrook J. Protein folding in the cell. Nature. 1992;355:33–45. - PubMed
-
- Gyrd-Hansen M, Nylandsted J, Jaattela M. Heat shock protein 70 promotes cancer cell viability by safeguarding lysosomal integrity. Cell Cycle. 2004;3:1484–1485. - PubMed
-
- Netzer WJ, Hartl FU. Protein folding in the cytosol: chaperonin-dependent and -independent mechanisms. Trends Biochem Sci. 1998;23:68–73. - PubMed
-
- Nylandsted J, Brand K, Jaattela M. Heat shock protein 70 is required for the survival of cancer cells. Annals of the New York Academy of Sciences. 2000;926:122–125. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

