Effects of fatty acid amide hydrolase inhibition on neuronal responses to nicotine, cocaine and morphine in the nucleus accumbens shell and ventral tegmental area: involvement of PPAR-alpha nuclear receptors
- PMID: 20477753
- PMCID: PMC3167063
- DOI: 10.1111/j.1369-1600.2010.00222.x
Effects of fatty acid amide hydrolase inhibition on neuronal responses to nicotine, cocaine and morphine in the nucleus accumbens shell and ventral tegmental area: involvement of PPAR-alpha nuclear receptors
Abstract
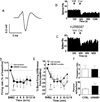
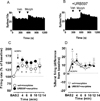
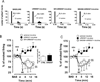
The endocannabinoid system regulates neurotransmission in brain regions relevant to neurobiological and behavioral actions of addicting drugs. We recently demonstrated that inhibition by URB597 of fatty acid amide hydrolase (FAAH), the main enzyme that degrades the endogenous cannabinoid N-acylethanolamine (NAE) anandamide and the endogenous non-cannabinoid NAEs oleoylethanolamide and palmitoylethanolamide, blocks nicotine-induced excitation of ventral tegmental area (VTA) dopamine (DA) neurons and DA release in the shell of the nucleus accumbens (ShNAc), as well as nicotine-induced drug self-administration, conditioned place preference and relapse in rats. Here, we studied whether effects of FAAH inhibition on nicotine-induced changes in activity of VTA DA neurons were specific for nicotine or extended to two drugs of abuse acting through different mechanisms, cocaine and morphine. We also evaluated whether FAAH inhibition affects nicotine-, cocaine- or morphine-induced actions in the ShNAc. Experiments involved single-unit electrophysiological recordings from DA neurons in the VTA and medium spiny neurons in the ShNAc in anesthetized rats. We found that URB597 blocked effects of nicotine and cocaine in the ShNAc through activation of both surface cannabinoid CB1-receptors and alpha-type peroxisome proliferator-activated nuclear receptor. URB597 did not alter the effects of either cocaine or morphine on VTA DA neurons. These results show that the blockade of nicotine-induced excitation of VTA DA neurons, which we previously described, is selective for nicotine and indicate novel mechanisms recruited to regulate the effects of addicting drugs within the ShNAc of the brain reward system.
Figures


Similar articles
-
Endogenous fatty acid ethanolamides suppress nicotine-induced activation of mesolimbic dopamine neurons through nuclear receptors.J Neurosci. 2008 Dec 17;28(51):13985-94. doi: 10.1523/JNEUROSCI.3221-08.2008. J Neurosci. 2008. PMID: 19091987 Free PMC article.
-
Blockade of nicotine reward and reinstatement by activation of alpha-type peroxisome proliferator-activated receptors.Biol Psychiatry. 2011 Apr 1;69(7):633-41. doi: 10.1016/j.biopsych.2010.07.009. Biol Psychiatry. 2011. PMID: 20801430 Free PMC article.
-
Inhibition of anandamide hydrolysis by cyclohexyl carbamic acid 3'-carbamoyl-3-yl ester (URB597) reverses abuse-related behavioral and neurochemical effects of nicotine in rats.J Pharmacol Exp Ther. 2008 Nov;327(2):482-90. doi: 10.1124/jpet.108.142224. Epub 2008 Aug 25. J Pharmacol Exp Ther. 2008. PMID: 18725543 Free PMC article.
-
Localization of brain reinforcement mechanisms: intracranial self-administration and intracranial place-conditioning studies.Behav Brain Res. 1999 Jun;101(2):129-52. doi: 10.1016/s0166-4328(99)00022-4. Behav Brain Res. 1999. PMID: 10372570 Review.
-
Lateral hypothalamic orexin/hypocretin neurons: A role in reward-seeking and addiction.Brain Res. 2010 Feb 16;1314:74-90. doi: 10.1016/j.brainres.2009.09.106. Epub 2009 Oct 6. Brain Res. 2010. PMID: 19815001 Free PMC article. Review.
Cited by
-
Beta-caryophyllene inhibits cocaine addiction-related behavior by activation of PPARα and PPARγ: repurposing a FDA-approved food additive for cocaine use disorder.Neuropsychopharmacology. 2021 Mar;46(4):860-870. doi: 10.1038/s41386-020-00885-4. Epub 2020 Oct 17. Neuropsychopharmacology. 2021. PMID: 33069159 Free PMC article.
-
Potential of Cannabinoid Receptor Ligands as Treatment for Substance Use Disorders.CNS Drugs. 2019 Oct;33(10):1001-1030. doi: 10.1007/s40263-019-00664-w. CNS Drugs. 2019. PMID: 31549358 Free PMC article. Review.
-
Modulation of the endocannabinoid system: vulnerability factor and new treatment _target for stimulant addiction.Front Psychiatry. 2013 Sep 23;4:109. doi: 10.3389/fpsyt.2013.00109. Front Psychiatry. 2013. PMID: 24069004 Free PMC article. Review.
-
The peroxisome proliferator-activated receptor alpha agonist fenofibrate attenuates alcohol self-administration in rats.Neuropharmacology. 2017 Apr;116:364-370. doi: 10.1016/j.neuropharm.2017.01.007. Epub 2017 Jan 11. Neuropharmacology. 2017. PMID: 28088358 Free PMC article.
-
Role of the endogenous cannabinoid system in nicotine addiction: novel insights.Front Psychiatry. 2015 Mar 25;6:41. doi: 10.3389/fpsyt.2015.00041. eCollection 2015. Front Psychiatry. 2015. PMID: 25859226 Free PMC article. Review.
References
-
- Adamczyk P, McCreary AC, Przegalinski E, Mierzejewski P, Bienkowski P, Filip M. The effects of fatty acid amide hydrolase inhibitors on maintenance of cocaine and food self-administration and on reinstatement of cocaine-seeking and food-taking behavior in rats. J Physiol Pharmacol. 2009;60:119–125. - PubMed
-
- Bickerdike MJ, Abercrombie ED. Striatal acetylcholine release correlates with behavioral sensitization in rats withdrawn from chronic amphetamine. J Pharmacol Exp Ther. 1997;282:818–826. - PubMed
-
- Cossu G, Ledent C, Fattore L, Imperato A, Bohme GA, Parmentier M, Fratta W. Cannabinoid CB1 receptor knockout mice fail to self-administer morphine but not other drugs of abuse. Behav Brain Res. 2001;118:61–65. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

