A genetic determinant of the striatal dopamine response to alcohol in men
- PMID: 20479755
- PMCID: PMC2925052
- DOI: 10.1038/mp.2010.56
A genetic determinant of the striatal dopamine response to alcohol in men
Abstract
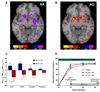
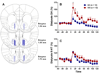
Excessive alcohol use, a major cause of morbidity and mortality, is less well understood than other addictive disorders. Dopamine release in ventral striatum is a common element of drug reward, but alcohol has an unusually complex pharmacology, and humans vary greatly in their alcohol responses. This variation is related to genetic susceptibility for alcoholism, which contributes more than half of alcoholism risk. Here, we report that a functional OPRM1 A118G polymorphism is a major determinant of striatal dopamine responses to alcohol. Social drinkers recruited based on OPRM1 genotype were challenged in separate sessions with alcohol and placebo under pharmacokinetically controlled conditions, and examined for striatal dopamine release using positron emission tomography and [(11)C]-raclopride displacement. A striatal dopamine response to alcohol was restricted to carriers of the minor 118G allele. To directly establish the causal role of OPRM1 A118G variation, we generated two humanized mouse lines, carrying the respective human sequence variant. Brain microdialysis showed a fourfold greater peak dopamine response to an alcohol challenge in h/mOPRM1-118GG than in h/mOPRM1-118AA mice. OPRM1 A118G variation is a genetic determinant of dopamine responses to alcohol, a mechanism by which it likely modulates alcohol reward.
Conflict of interest statement
None of the authors has any competing interest, financial or otherwise.
Figures

Similar articles
-
A pharmacogenetic determinant of mu-opioid receptor antagonist effects on alcohol reward and consumption: evidence from humanized mice.Biol Psychiatry. 2015 May 15;77(10):850-8. doi: 10.1016/j.biopsych.2014.08.021. Epub 2014 Sep 8. Biol Psychiatry. 2015. PMID: 25442002
-
Receptor Reserve Moderates Mesolimbic Responses to Opioids in a Humanized Mouse Model of the OPRM1 A118G Polymorphism.Neuropsychopharmacology. 2015 Oct;40(11):2614-22. doi: 10.1038/npp.2015.109. Epub 2015 Apr 16. Neuropsychopharmacology. 2015. PMID: 25881115 Free PMC article.
-
Increased ethanol drinking in "humanized" mice expressing the mu opioid receptor A118G polymorphism are mediated through sex-specific mechanisms.Brain Res Bull. 2018 Apr;138:12-19. doi: 10.1016/j.brainresbull.2017.07.017. Epub 2017 Aug 2. Brain Res Bull. 2018. PMID: 28780411 Free PMC article.
-
Association of µ-opioid receptor (OPRM1) gene polymorphism with response to naltrexone in alcohol dependence: a systematic review and meta-analysis.Addict Biol. 2012 May;17(3):505-12. doi: 10.1111/j.1369-1600.2012.00442.x. Addict Biol. 2012. PMID: 22515274 Review.
-
Influence of the OPRM1 A118G polymorphism on alcohol-induced euphoria, risk for alcoholism and the clinical efficacy of naltrexone.Pharmacogenomics. 2012 Jul;13(10):1161-72. doi: 10.2217/pgs.12.99. Pharmacogenomics. 2012. PMID: 22909206 Review.
Cited by
-
Opioid pharmacogenetics of alcohol addiction.Cold Spring Harb Perspect Med. 2013 Jul 1;3(7):a012203. doi: 10.1101/cshperspect.a012203. Cold Spring Harb Perspect Med. 2013. PMID: 23729643 Free PMC article. Review.
-
The genetic basis of addictive disorders.Psychiatr Clin North Am. 2012 Jun;35(2):495-519. doi: 10.1016/j.psc.2012.03.010. Psychiatr Clin North Am. 2012. PMID: 22640768 Free PMC article.
-
Genetic and environmental determinants of stress responding.Alcohol Res. 2012;34(4):484-94. Alcohol Res. 2012. PMID: 23584114 Free PMC article. Review.
-
Human laboratory paradigms in alcohol research.Alcohol Clin Exp Res. 2012 Jun;36(6):972-83. doi: 10.1111/j.1530-0277.2011.01704.x. Epub 2012 Feb 6. Alcohol Clin Exp Res. 2012. PMID: 22309888 Free PMC article. Review.
-
Exploring Pharmacological Functions of Alternatively Spliced Variants of the Mu Opioid Receptor Gene, Oprm1, via Gene-_targeted Animal Models.Int J Mol Sci. 2022 Mar 10;23(6):3010. doi: 10.3390/ijms23063010. Int J Mol Sci. 2022. PMID: 35328429 Free PMC article. Review.
References
-
- Rehm J, Mathers C, Popova S, Thavorncharoensap M, Teerawattananon Y, Patra J. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. The Lancet. 2009;373(9682):2223–2233. - PubMed
-
- Bouza C, Angeles M, Munoz A, Amate JM. Efficacy and safety of naltrexone and acamprosate in the treatment of alcohol dependence: a systematic review. Addiction. 2004;99(7):811–828. - PubMed
-
- Heilig M, Egli M. Pharmacological treatment of alcohol dependence: _target symptoms and _target mechanisms. Pharm Therap. 2006;111(3):855–876. - PubMed
-
- Spanagel R. Alcoholism: A Systems Approach From Molecular Physiology to Addictive Behavior. Physiol Rev. 2009;89(2):649–705. - PubMed
-
- Schuckit MA. Vulnerability Factors for Alcoholism. In: Davis KL, Charney D, Coyle JT, Nemeroff C, editors. Neuropsychopharmacology: The fifth generation of progress. 41 ed. Philadelphia: Lippincott Williams & Wilkins; 2002. pp. 1399–1412.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

