Renal lipid metabolism and lipotoxicity
- PMID: 20489613
- PMCID: PMC3080272
- DOI: 10.1097/MNH.0b013e32833aa4ac
Renal lipid metabolism and lipotoxicity
Abstract
Purpose of review: Lipid accumulation in nonadipose tissues is increasingly recognized to contribute to organ injury through a process termed lipotoxicity, but whether this process occurs in the kidney is still uncertain. This article briefly summarizes the normal role of lipids in renal physiology and the current evidence linking excess lipids and lipotoxicity to renal dysfunction.
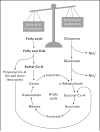
Recent findings: Evidence suggesting that renal lipid accumulation and lipotoxicity may lead to kidney dysfunction has mounted significantly over recent years. Abnormal renal lipid content has been described in a number of animal models and has been successfully manipulated using pharmacologic or genetic strategies. There is some heterogeneity among studies with regard to the mechanisms, consequences, and localization of lipid accumulation in the kidney, explainable at least in part by inherent differences between animal models. The relevance of these findings for human pathophysiology remains to be established.
Summary: Current knowledge on renal lipid physiology and pathophysiology is insufficient, but provides a strong foundation and incentive for further exploration. The future holds significant challenges in this area, especially with regard to applicability of research findings to the human kidney in vivo, but also the opportunity to transform our understanding of an array of kidney disorders.
Figures


Similar articles
-
Renal lipotoxicity: Insights from experimental models.Clin Exp Pharmacol Physiol. 2021 Dec;48(12):1579-1588. doi: 10.1111/1440-1681.13556. Epub 2021 Sep 23. Clin Exp Pharmacol Physiol. 2021. PMID: 34314523 Review.
-
Lipotoxicity and Diabetic Nephropathy: Novel Mechanistic Insights and Therapeutic Opportunities.Int J Mol Sci. 2020 Apr 10;21(7):2632. doi: 10.3390/ijms21072632. Int J Mol Sci. 2020. PMID: 32290082 Free PMC article. Review.
-
Altered renal lipid metabolism and renal lipid accumulation in human diabetic nephropathy.J Lipid Res. 2014 Mar;55(3):561-72. doi: 10.1194/jlr.P040501. Epub 2013 Dec 26. J Lipid Res. 2014. PMID: 24371263 Free PMC article.
-
Sex differences in obesity-induced renal lipid accumulation revealed by lipidomics: a role of adiponectin/AMPK axis.Biol Sex Differ. 2023 Sep 28;14(1):63. doi: 10.1186/s13293-023-00543-6. Biol Sex Differ. 2023. PMID: 37770988 Free PMC article.
-
Lipotoxicity: when tissues overeat.Curr Opin Lipidol. 2003 Jun;14(3):281-7. doi: 10.1097/00041433-200306000-00008. Curr Opin Lipidol. 2003. PMID: 12840659 Review.
Cited by
-
Role of Bile Acid Receptors in the Development and Function of Diabetic Nephropathy.Kidney Int Rep. 2024 Aug 10;9(11):3116-3133. doi: 10.1016/j.ekir.2024.08.002. eCollection 2024 Nov. Kidney Int Rep. 2024. PMID: 39534198 Free PMC article. Review.
-
PGC-1α-mediated imbalance of mitochondria-lipid droplet homeostasis in neomycin-induced ototoxicity and nephrotoxicity.Acta Pharm Sin B. 2024 Oct;14(10):4413-4430. doi: 10.1016/j.apsb.2024.05.024. Epub 2024 Jun 4. Acta Pharm Sin B. 2024. PMID: 39525588 Free PMC article.
-
Lipid metabolism disorders and albuminuria risk: insights from National Health and Nutrition Examination Survey 2001-2018 and Mendelian randomization analyses.Ren Fail. 2024 Dec;46(2):2420841. doi: 10.1080/0886022X.2024.2420841. Epub 2024 Nov 3. Ren Fail. 2024. PMID: 39491271 Free PMC article.
-
Quantitative assessment of renal steatosis in patients with type 2 diabetes mellitus using the iterative decomposition of water and fat with echo asymmetry and least squares estimation quantification sequence imaging: repeatability and clinical implications.Quant Imaging Med Surg. 2024 Oct 1;14(10):7341-7352. doi: 10.21037/qims-24-330. Epub 2024 Sep 23. Quant Imaging Med Surg. 2024. PMID: 39429570 Free PMC article.
-
Fenofibrate attenuates renal lipotoxicity in uninephrectomized mice with high-fat diet-induced obesity.J Bras Nefrol. 2024 Oct-Dec;46(4):e20230148. doi: 10.1590/2175-8239-JBN-2023-0148en. J Bras Nefrol. 2024. PMID: 39412511 Free PMC article.
References
-
- Olofsson SO, Bostrom P, Andersson L, et al. Lipid droplets as dynamic organelles connecting storage and efflux of lipids. Biochim Biophys Acta. 2009;1791:448–458. [An overview of the novel and fascinating field of lipid droplet biology, including a discussion of the potential role of lipid droplets in insulin resistance and atherosclerosis.] - PubMed
-
- Meex RC, Schrauwen P, Hesselink MK. Modulation of myocellular fat stores: lipid droplet dynamics in health and disease. Am J Physiol Regul Integr Comp Physiol. 2009;297:R913–R924. [An excellent review of lipid droplet dynamics and their potential role in skeletal muscle insulin sensitivity.] - PubMed
-
- Schaffer JE. Lipotoxicity: when tissues overeat. Curr Opin Lipidol. 2003;14:281–287. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

