Genetic labeling does not detect epithelial-to-mesenchymal transition of cholangiocytes in liver fibrosis in mice
- PMID: 20546735
- PMCID: PMC2930026
- DOI: 10.1053/j.gastro.2010.05.005
Genetic labeling does not detect epithelial-to-mesenchymal transition of cholangiocytes in liver fibrosis in mice
Abstract
Background & aims: Chronic injury changes the fate of certain cellular populations, inducing epithelial cells to generate fibroblasts by epithelial-to-mesenchymal transition (EMT) and mesenchymal cells to generate epithelial cells by mesenchymal-to-epithelial transition (MET). Although contribution of EMT/MET to embryogenesis, renal fibrosis, and lung fibrosis is well documented, role of EMT/MET in liver fibrosis is unclear. We determined whether cytokeratin-19 positive (K19(+)) cholangiocytes give rise to myofibroblasts (EMT) and/or whether glial fibrillary acidic protein positive (GFAP(+)) hepatic stellate cells (HSCs) can express epithelial markers (MET) in response to experimental liver injury.
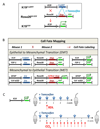
Methods: EMT was studied with Cre-loxP system to map cell fate of K19(+) cholangiocytes in K19(YFP) or fibroblast-specific protein-1 (FSP-1)(YFP) mice, generated by crossing tamoxifen-inducible K19(CreERT) mice or FSP-1(Cre) mice with Rosa26(f/f-YFP) mice. MET of GFAP(+) HSCs was studied in GFAP(GFP) mice. Mice were subjected to bile duct ligation or CCl(4)-liver injury, and livers were analyzed for expression of mesodermal and epithelial markers.
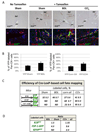
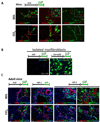
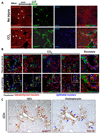
Results: On Cre-loxP recombination, >40% of genetically labeled K19(+) cholangiocytes expressed yellow fluorescent protein (YFP). All mice developed liver fibrosis. However, specific immunostaining of K19(YFP) cholangiocytes showed no expression of EMT markers alpha-smooth muscle actin, desmin, or FSP-1. Moreover, cells genetically labeled by FSP-1(YFP) expression did not coexpress cholangiocyte markers K19 or E-cadherin. Genetically labeled GFAP(GFP) HSCs did not express epithelial or liver progenitor markers in response to liver injury.
Conclusion: EMT of cholangiocytes identified by genetic labeling does not contribute to hepatic fibrosis in mice. Likewise, GFAP(Cre)-labeled HSCs showed no coexpression of epithelial markers, providing no evidence for MET in HSCs in response to fibrogenic liver injury.
Copyright © 2010 AGA Institute. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Disclosures – the authors have no conflict of interest.
Figures


Comment in
-
Epithelial-to-mesenchymal transition in liver fibrosis: dead or alive?Gastroenterology. 2010 Sep;139(3):722-5. doi: 10.1053/j.gastro.2010.07.015. Epub 2010 Aug 1. Gastroenterology. 2010. PMID: 20682361 No abstract available.
Similar articles
-
Epithelial-to-mesenchymal transition in liver fibrosis: dead or alive?Gastroenterology. 2010 Sep;139(3):722-5. doi: 10.1053/j.gastro.2010.07.015. Epub 2010 Aug 1. Gastroenterology. 2010. PMID: 20682361 No abstract available.
-
Lineage tracing demonstrates no evidence of cholangiocyte epithelial-to-mesenchymal transition in murine models of hepatic fibrosis.Hepatology. 2011 May;53(5):1685-95. doi: 10.1002/hep.24206. Hepatology. 2011. PMID: 21520179 Free PMC article.
-
Mesodermal mesenchymal cells give rise to myofibroblasts, but not epithelial cells, in mouse liver injury.Hepatology. 2014 Jul;60(1):311-22. doi: 10.1002/hep.27035. Epub 2014 Apr 28. Hepatology. 2014. PMID: 24488807 Free PMC article.
-
Wnt signaling in liver fibrosis: progress, challenges and potential directions.Biochimie. 2013 Dec;95(12):2326-35. doi: 10.1016/j.biochi.2013.09.003. Epub 2013 Sep 13. Biochimie. 2013. PMID: 24036368 Review.
-
Study on the relationship between hepatic fibrosis and epithelial-mesenchymal transition in intrahepatic cells.Biomed Pharmacother. 2020 Sep;129:110413. doi: 10.1016/j.biopha.2020.110413. Epub 2020 Jun 20. Biomed Pharmacother. 2020. PMID: 32570119 Review.
Cited by
-
Immune Inhibitory Properties and Therapeutic Prospects of Transforming Growth Factor-Beta and Interleukin 10 in Autoimmune Hepatitis.Dig Dis Sci. 2022 Apr;67(4):1163-1186. doi: 10.1007/s10620-021-06968-6. Epub 2021 Apr 9. Dig Dis Sci. 2022. PMID: 33835375 Review.
-
Inflammation-mediated genetic and epigenetic alterations drive cancer development in the neighboring epithelium upon stromal abrogation of TGF-β signaling.PLoS Genet. 2013;9(2):e1003251. doi: 10.1371/journal.pgen.1003251. Epub 2013 Feb 7. PLoS Genet. 2013. PMID: 23408900 Free PMC article.
-
Col1A1 production and apoptotic resistance in TGF-β1-induced epithelial-to-mesenchymal transition-like phenotype of 603B cells.PLoS One. 2012;7(12):e51371. doi: 10.1371/journal.pone.0051371. Epub 2012 Dec 7. PLoS One. 2012. PMID: 23236489 Free PMC article.
-
Bone marrow-derived fibrocytes contribute to liver fibrosis.Exp Biol Med (Maywood). 2015 Jun;240(6):691-700. doi: 10.1177/1535370215584933. Epub 2015 May 12. Exp Biol Med (Maywood). 2015. PMID: 25966982 Free PMC article. Review.
-
Portal Fibroblasts in Biliary Fibrosis.Curr Pathobiol Rep. 2014 Dec 1;2(4):185-190. doi: 10.1007/s40139-014-0054-y. Curr Pathobiol Rep. 2014. PMID: 25599013 Free PMC article.
References
-
- Parola M, Marra F, Pinzani M. Myofibroblast - like cells and liver fibrogenesis: Emerging concepts in a rapidly moving scenario. Mol Aspects Med. 2008;29:58–66. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

