Renal failure in mice with Gsalpha deletion in juxtaglomerular cells
- PMID: 20551626
- PMCID: PMC2914394
- DOI: 10.1159/000314635
Renal failure in mice with Gsalpha deletion in juxtaglomerular cells
Abstract
Background: Mice with deletion of Gsalpha in renin-producing cells (RC/FF mice) have been shown to have greatly reduced renin production and lack of responsiveness of renin secretion to acute stimuli. In addition, young RC/FF mice are hypotensive and have a vasopressin-resistant concentrating defect. In the present study we have determined the long-term effect on renal function, blood pressure, and renal pathology in this low renin and diuretic mouse model.
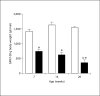
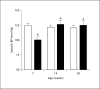
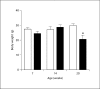
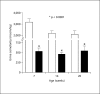
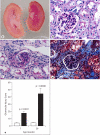
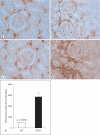
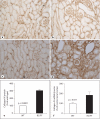
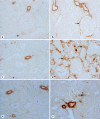
Methods and results: Urine osmolarity of RC/FF mice was decreased in all age groups. GFR measured at 7, 14 and 20 weeks of age declined progressively. Single nephron GFR similarly declined while fractional proximal fluid absorption was maintained. Expression levels of extracellular matrix proteins (collagen I, IV and fibronectin) and alpha-smooth muscle actin were increased in kidneys of RC/FF mice at 20 weeks, and this was accompanied by focal segmental glomerulosclerosis and periglomerular interstitial fibrosis. RC/FF mice showed a progressive reduction of body weight, an increase in urine albumin excretion, and an increase of blood pressure with aging.
Conclusion: A chronic reduction of renin production in mice may be a risk factor in its own right, and does not protect renal function against the profibrotic influence of a chronically elevated urine flow.
Copyright 2010 S. Karger AG, Basel.
Figures




Similar articles
-
The molecular basis of increased glomerulosclerosis after blockade of the renin angiotensin system in growth hormone transgenic mice.Mol Med. 1994 Nov;1(1):104-15. Mol Med. 1994. PMID: 8790606 Free PMC article.
-
Regulation of renin in mice with Cre recombinase-mediated deletion of G protein Gsalpha in juxtaglomerular cells.Am J Physiol Renal Physiol. 2007 Jan;292(1):F27-37. doi: 10.1152/ajprenal.00193.2006. Epub 2006 Jul 5. Am J Physiol Renal Physiol. 2007. PMID: 16822937
-
Interference with Gsα-Coupled Receptor Signaling in Renin-Producing Cells Leads to Renal Endothelial Damage.J Am Soc Nephrol. 2017 Dec;28(12):3479-3489. doi: 10.1681/ASN.2017020173. Epub 2017 Aug 3. J Am Soc Nephrol. 2017. PMID: 28775003 Free PMC article.
-
In vitro study of the juxtaglomerular apparatus and its implications in the chronic kidney disease.Hypertension. 2015 May;65(5):970-5. doi: 10.1161/HYPERTENSIONAHA.114.04365. Epub 2015 Feb 2. Hypertension. 2015. PMID: 25646294 Review. No abstract available.
-
Flexible and multifaceted: the plasticity of renin-expressing cells.Pflugers Arch. 2022 Aug;474(8):799-812. doi: 10.1007/s00424-022-02694-8. Epub 2022 May 5. Pflugers Arch. 2022. PMID: 35511367 Free PMC article. Review.
Cited by
-
RAS-Mediated Adaptive Mechanisms in Cardiovascular Tissues: Confounding Factors of RAS Blockade Therapy and Alternative Approaches.Cardiorenal Med. 2012 Dec;2(4):268-280. doi: 10.1159/000343456. Epub 2012 Oct 27. Cardiorenal Med. 2012. PMID: 23381810 Free PMC article.
-
Smooth muscle BK channel activity influences blood pressure independent of vascular tone in mice.J Physiol. 2014 Jun 15;592(12):2563-74. doi: 10.1113/jphysiol.2014.272880. Epub 2014 Mar 31. J Physiol. 2014. PMID: 24687584 Free PMC article.
-
Fate and plasticity of renin precursors in development and disease.Pediatr Nephrol. 2014 Apr;29(4):721-6. doi: 10.1007/s00467-013-2688-0. Epub 2013 Dec 15. Pediatr Nephrol. 2014. PMID: 24337407 Free PMC article. Review.
-
Local renal circadian clocks control fluid-electrolyte homeostasis and BP.J Am Soc Nephrol. 2014 Jul;25(7):1430-9. doi: 10.1681/ASN.2013060641. Epub 2014 Mar 20. J Am Soc Nephrol. 2014. PMID: 24652800 Free PMC article.
-
Activation of the renal GLP-1R leads to expression of Ren1 in the renal vascular tree.Endocrinol Diabetes Metab. 2021 Mar 19;4(3):e00234. doi: 10.1002/edm2.234. eCollection 2021 Jul. Endocrinol Diabetes Metab. 2021. PMID: 34277961 Free PMC article.
References
-
- Chen L, Kim SM, Oppermann M, et al. Regulation of renin in mice with Cre recombinase-mediated deletion of G protein Gsα in juxtaglomerular cells. Am J Physiol Renal Physiol. 2007;292:F27–F37. - PubMed
-
- Maschio G, Alberti D, Locatelli F, et al. Angiotensin-converting enzyme inhibitors and kidney protection: the AIPRI trial. The ACE Inhibition in Progressive Renal Insufficiency (AIPRI) Study Group. J Cardiovasc Pharmacol. 1999;33(suppl 1):S16–S20. S41–S43. - PubMed
-
- Klahr S. Urinary tract obstruction. Semin Nephrol. 2001;21:133–145. - PubMed
-
- Mervaala EM, Muller DN, Park JK, et al. Monocyte infiltration and adhesion molecules in a rat model of high human renin hypertension. Hypertension. 1999;33:389–395. - PubMed
-
- Yang G, Merrill DC, Thompson MW, Robillard JE, Sigmund CD. Functional expression of the human angiotensinogen gene in transgenic mice. J Biol Chem. 1994;269:32497–32502. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

