The role of EZH2 and DNA methylation in the silencing of the tumour suppressor RUNX3 in colorectal cancer
- PMID: 20631058
- PMCID: PMC2930806
- DOI: 10.1093/carcin/bgq147
The role of EZH2 and DNA methylation in the silencing of the tumour suppressor RUNX3 in colorectal cancer
Abstract
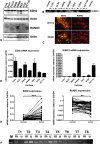
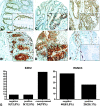
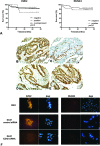
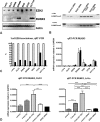
In gastric cancer, a new epigenetic mechanism of tumour suppressor loss has been suggested where the histone methyltransferase enhancer of zeste homolog 2 (EZH2) is responsible for loss of expression of RUNX3. This is consistent with EZH2 upregulation in multiple cancer types being associated with poor prognosis. We investigated whether EZH2 influences the expression of RUNX3 in colorectal cancer (CRC) and whether this is independent of methylation. We determined protein and messenger RNA (mRNA) levels of EZH2 and RUNX3 and assessed RUNX3 methylation with methylation-specific polymerase chain reaction using 72 human CRCs and 8 CRC cell lines. We assessed the effect of efficient RNA interference-mediated knockdown of EZH2 on RUNX3 levels, cell viability and H3K27 trimethylation of the RUNX3 promoter using chromatin immunoprecipitation. Despite higher levels of EZH2 and lower levels of RUNX3 in CRC specimens in general, no inverse correlation between EZH2 and RUNX3 in paired samples was found arguing against a major role for histone methylation in silencing RUNX3 in CRC. Conversely, downregulation of RUNX3 mRNA in the same tumours was associated with RUNX3 DNA methylation (P < 0.05). In cell lines, knockdown of EZH2 removed the repressive chromatin marks from RUNX3 but did not result in RUNX3 re-expression. However, it prevented the re-silencing of RUNX3 after the removal of demethylating agents. In conclusion, DNA methylation is primarily responsible for the transcriptional silencing of RUNX3 in CRC, but EZH2 and histone methylation are necessary for its methylation-dependent re-silencing after the removal of demethylating agents. These results would predict that inhibitors of EZH2 and histone methylation would enhance the effects of demethylating agents in cancer therapy.
Figures
Similar articles
-
Enhancer of zeste homologue 2 (EZH2) down-regulates RUNX3 by increasing histone H3 methylation.J Biol Chem. 2008 Jun 20;283(25):17324-32. doi: 10.1074/jbc.M800224200. Epub 2008 Apr 22. J Biol Chem. 2008. PMID: 18430739 Free PMC article.
-
EZH2 Mediates epigenetic silencing of neuroblastoma suppressor genes CASZ1, CLU, RUNX3, and NGFR.Cancer Res. 2012 Jan 1;72(1):315-24. doi: 10.1158/0008-5472.CAN-11-0961. Epub 2011 Nov 8. Cancer Res. 2012. PMID: 22068036 Free PMC article.
-
Regulation of the expression of claudin 23 by the enhancer of zeste 2 polycomb group protein in colorectal cancer.Mol Med Rep. 2015 Jul;12(1):728-36. doi: 10.3892/mmr.2015.3378. Epub 2015 Feb 19. Mol Med Rep. 2015. PMID: 25695204
-
Roles of the EZH2 histone methyltransferase in cancer epigenetics.Mutat Res. 2008 Dec 1;647(1-2):21-9. doi: 10.1016/j.mrfmmm.2008.07.010. Epub 2008 Aug 3. Mutat Res. 2008. PMID: 18723033 Review.
-
EZH2 methyltransferase and H3K27 methylation in breast cancer.Int J Biol Sci. 2012;8(1):59-65. doi: 10.7150/ijbs.8.59. Epub 2011 Nov 18. Int J Biol Sci. 2012. PMID: 22211105 Free PMC article. Review.
Cited by
-
The interplay between DNA and histone methylation: molecular mechanisms and disease implications.EMBO Rep. 2021 May 5;22(5):e51803. doi: 10.15252/embr.202051803. Epub 2021 Apr 12. EMBO Rep. 2021. PMID: 33844406 Free PMC article. Review.
-
GSK343 induces programmed cell death through the inhibition of EZH2 and FBP1 in osteosarcoma cells.Cancer Biol Ther. 2020;21(3):213-222. doi: 10.1080/15384047.2019.1680061. Epub 2019 Oct 25. Cancer Biol Ther. 2020. PMID: 31651209 Free PMC article.
-
CircRNA LRP6 promotes the development of osteosarcoma via negatively regulating KLF2 and APC levels.Am J Transl Res. 2019 Jul 15;11(7):4126-4138. eCollection 2019. Am J Transl Res. 2019. PMID: 31396323 Free PMC article.
-
RUNX3 enhances TRAIL-induced apoptosis by upregulating DR5 in colorectal cancer.Oncogene. 2019 May;38(20):3903-3918. doi: 10.1038/s41388-019-0693-x. Epub 2019 Jan 28. Oncogene. 2019. PMID: 30692634
-
Increased EZH2 expression during the adenoma-carcinoma sequence in colorectal cancer.Oncol Lett. 2018 Oct;16(4):5275-5281. doi: 10.3892/ol.2018.9240. Epub 2018 Jul 31. Oncol Lett. 2018. PMID: 30214616 Free PMC article.
References
-
- Varambally S, et al. The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature. 2002;419:624–629. - PubMed
-
- Vire E, et al. The Polycomb group protein EZH2 directly controls DNA methylation. Nature. 2006;439:871–874. - PubMed
-
- Schlesinger Y, et al. Polycomb-mediated methylation on Lys27 of histone H3 pre-marks genes for de novo methylation in cancer. Nat. Genet. 2007;39:232–236. - PubMed
-
- McGarvey KM, et al. DNA methylation and complete transcriptional silencing of cancer genes persist after depletion of EZH2. Cancer Res. 2007;67:5097–5102. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

